ABSTRACT
Extracorporeal carbon dioxide removal (ECCO2R) uses mechanical systems to treat hypercapnic respiratory failure. Its utility has been investigated in acute respiratory distress syndrome (ARDS), acute exacerbations of chronic obstructive pulmonary disease (COPD), and status asthmaticus, and as a bridge to lung transplant. In this review, we discuss how it works, why it should help, and current evidence supporting its use.
While ECCO2R may help facilitate low tidal volume ventilation in ARDS, evidence that it improves the survival rate is as yet wanting.
Similarly, although this therapy appears promising in other indications, evidence is still sparse.
The risks associated with ECCO2R, including hemorrhage, must be weighed against its purported clinical benefits.
At this time, the use of ECCO2R, promising as it is, should be explored within the confines of clinical research with a view to improving its safety and efficacy.
Extracorporeal carbon dioxide removal (ECCO2R) is similar to extracorporeal membrane oxygenation (ECMO) in that it involves shunting blood through a membrane device. But unlike ECMO, it does not provide significant oxygenation. The primary purpose is to remove carbon dioxide. Compared with ECMO, ECCO2R can be used with lower blood flow rates and smaller cannulas.1 It may also be less expensive and easier to implement.
ECCO2R has been studied in various pulmonary diseases, eg, acute respiratory distress syndrome (ARDS), chronic obstructive pulmonary disease (COPD),2 and asthma,3 and as a bridge to lung transplant.4 Currently, only one ECCO2R device is approved by the US Food and Drug Administration, for use of up to 5 days.
As use of ECCO2R becomes more widespread, physicians will need to get more familiar with it. Extracorporeal life support technologies such as ECMO and ECCO2R require highly specialized training and technology.5 The Extracorporeal Life Support Organization currently lists 351 ECMO-certified centers in the United States.6
This article, a primer for clinicians, provides an overview of ECCO2R including the rationale for its use, how it works, and evidence of its benefits.
WHY REMOVE CARBON DIOXIDE?
Hypercapnia and ensuing acidosis have detrimental effects on multiple body systems. Hypercapnia decreases myocardial contractility and increases pulmonary vasoconstriction, which could worsen right ventricular afterload, perpetuating right ventricular failure.7 Hypercapnic acidemia may also contribute to lung injury by increasing production of nitric oxide. But on the other hand, it mitigates lung injury by decreasing reactive oxygen species, ie, superoxides. Theoretical benefits of ECCO2R include the following:
Avoiding barotrauma. Normally, we get rid of carbon dioxide by breathing it out, and for a patient on a ventilator, to get rid of more carbon dioxide, we would have to turn up the tidal volume, the ventilation rate, or both. But increasing the tidal volume can cause barotrauma. ECCO2R allows us to keep the tidal volume low.
Slightly better oxygenation. As the arterial partial pressure of carbon dioxide goes down, the partial pressure of oxygen in the alveoli should go up according to the alveolar gas equation—whereby, basically, the alveolar partial pressure of oxygen equals the fraction of inspired oxygen minus the partial pressure of carbon dioxide. Also, ECCO2R, by correcting hypercapnia, allows the ventilation strategy to focus on oxygenation.8
CARBON DIOXIDE PHYSIOLOGY
Carbon dioxide is the product of metabolism within mitochondria.9 With the help of the enzyme carbonic anhydrase, mostly in red blood cells, it combines with water to form carbonic acid, which dissociates into bicarbonate and hydrogen ions. All of these reactions are reversible, and although there is far more bicarbonate than dissolved carbon dioxide circulating in the blood, bicarbonate can rapidly be converted back to carbon dioxide as the latter is removed from the blood through breathing, maintaining the partial pressure of carbon dioxide.
Carbon dioxide is transported in the blood both dissolved in plasma and bound to hemoglobin, but the blood’s carrying capacity for carbon dioxide is not limited by the hemoglobin concentration and binding capacity, as it is for oxygen. Compared with oxygen, carbon dioxide is more soluble and diffusible in the blood and has a linear hemoglobin dissociation curve that keeps going up, whereas that of oxygen reaches a plateau.
Given these differences in transport and dissociation physiology, carbon dioxide removal is more effective than oxygen delivery at lower blood-flows.
ECCO2R SYSTEMS
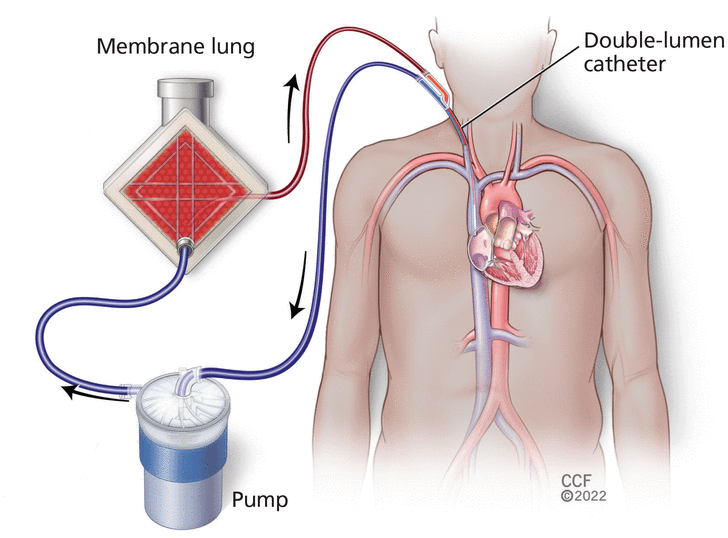
The typical ECCO2R setup (Figure 1) has 3 essential parts: the catheter or catheters, the membrane “lung,” and a pump (depending on the system) to circulate the blood.1 Because the equipment continues to evolve and get more complicated, the Extracorporeal Life Support Organization10 has developed standardized nomenclature to describe ECCO2R systems based on catheter function (drainage, reinfusion, or both), access site, size, and tip placement.
Basic venovenous extracorporeal carbon dioxide removal circuit.
ECCO2R systems are frequently classified as either venovenous or arteriovenous. Venovenous systems take blood from a vein and return it to a vein, sometimes the same vein. A pump generates the necessary flow, allowing cannulation through low-pressure venous vessels, often with a single, dual-lumen, bicaval catheter (Figure 1). Arteriovenous systems usually use 2 single-lumen cannulas, which take blood from an artery and return it to a vein, with the arterial pressure driving blood flow. Such pumpless systems, while causing less blood trauma, require adequate cardiac output and larger cannulas to maintain adequate blood flow.
ECCO2R systems typically operate at lower flow rates than ECMO systems, as low as 250 mL/minute in combined ECCO2R-hemodialysis systems.11
Table 1 compares key features of ECCO2R and ECMO systems.
Differences between extracorporeal carbon dioxide removal (ECCO2R) and extracorporeal membrane oxygenation (ECMO)
CARBON DIOXIDE REMOVAL MECHANISMS
ECCO2R systems use 2 main methods to remove carbon dioxide from blood.12
The membrane lung technique, the more common method, directly removes dissolved carbon dioxide by diffusion. Blood is circulated through microscopic channels on one side of a membrane, while gas without any carbon dioxide in it (the “sweep” gas) flows on the other side, generating a gradient so that the carbon dioxide diffuses across out of blood into this column of moving air. Other factors affecting carbon dioxide removal include the oxygen concentration in the sweep gas, the surface area of the membrane, and the rate of blood flow.
The respiratory dialysis technique removes carbon dioxide indirectly by removing bicarbonate ions by hemodialysis. By itself, this would make acidemia worse because it leaves the acidic hydrogen ions in place while removing the basic bicarbonate ions.13 To counter this deleterious effect, hydroxide and tris(hydroxy-methyl)aminomethane need to be infused, which can lead to hemolysis and arrhythmias. However, a 2020 study in pigs demonstrated the feasibility of this method by using a low-bicarbonate dialysate and avoiding blood acidification.14
Bicarbonate removal through ultrafiltration rather than hemodialysis has also been studied and can perform at lower blood flow rates than hemodialysis.15
ACUTE RESPIRATORY DISTRESS SYNDROME
About 10% of patients in intensive care units and 20% to 25% of patients on mechanical ventilators are there because they have ARDS.16 It is a heterogeneous syndrome caused by a dysregulated inflammatory response resulting in damage to the interface between the capillary endothelium and alveolar epithelium. This in turn leads to increased capillary permeability, noncardiogenic pulmonary edema, and decreased lung compliance.
A low-tidal-volume strategy is the cornerstone of ARDS management. Setting the ventilator to a lower tidal volume improves survival outcomes by reducing ventilator-induced lung injury, but it also leads to hypercapnia due to decreased alveolar ventilation. ECCO2R has undergone trials to see if it can alleviate this effect and permit low-tidal-volume ventilation (4–6 mL/kg predicted body weight) or even ultralow-tidal-volume ventilation (< 4 mL/kg predicted body weight).
While interest in using ECCO2R in ARDS dates back to the 1980s, investigation is ongoing.
The Xtravent study (Extrapulmonary Interventional Ventilatory Support in Severe Acute Respiratory Distress Syndrome),17 in 2013, compared ultralow-tidal-volume ventilation (about 3 mL/kg) plus ECCO2R vs about 6 mL/kg without ECCO2R in 79 patients with ARDS. Neither the number of ventilation-free days nor the mortality rate differed between the 2 treatment groups.
The SUPERNOVA trial (Strategy of Ultra-Protective Lung Ventilation with Extracorporeal CO2 Removal for new-Onset Moderate to Severe ARDS),18 in 2019, tested the feasibility of ECCO2R in maintaining ultralow tidal volume (4 mL/kg) in 95 patients with ARDS, 33 of whom were treated with a lower-powered carbon dioxide extraction machine and 62 with a higher-powered machine. Combining both groups, ultralow-tidal-volume ventilation was obtained by 24 hours in 82% of patients (64% with the low-powered machines and 92% with the high-powered machines, P < .001), with tidal volume, respiratory rate, minute ventilation, plateau pressure, and driving pressure significantly lower than at baseline; 69 patients (62%) survived to hospital discharge.19
The REST trial (Protective Ventilation With Veno-venous Lung Assist in Respiratory Failure),20 in 2021, found no statistically significant reduction in mortality at 90 days. A concern with this trial is that patients were recruited based on severity of hypoxemia rather than ARDS criteria, which only 60% met at enrollment. A significant number of patients may not have exhibited conventional ARDS physiologic patterns, such as increased alveolar dead-space fraction and decreased respiratory system compliance. Studies have shown that these 2 factors, rather than severity of hypoxemia, may be better entry criteria in ECCO2R studies.21 Also, the trial was stopped early due to futility and thus may have lacked power to detect a clinically important difference in mortality.
Acute respiratory distress syndrome due to COVID-19
ARDS due to COVID-19 can progress to hypercapnic respiratory failure,22 and ECCO2R has been used in this situation as well.
Akkanti et al23 reported that respiratory acidemia improved in 29 patients with its use, with peak effect within 24 hours. However, only 11 patients (38%) survived to hospital discharge. This high mortality rate (62%) is comparable to that in COVID-19 patients on ECMO (37% to 59%, worsening over time).24
Allescher et al25 studied the use of the Advanced Organ Support system (ADVOS), a combined ECCO2R-renal replacement-liver support system, in COVID-19 patients with mild to moderate ARDS. Multiple laboratory values improved (creatinine, blood urea nitrogen, pH, bicarbonate), but the in-hospital mortality rate was still 55%.
ACUTE EXACERBATION OF CHRONIC OBSTRUCTIVE PULMONARY DISEASE
COPD is defined by chronic inflammation of the airways, pulmonary parenchyma, and vasculature.26 Its natural course includes episodes of acute deterioration (exacerbations), with a reported intensive care unit mortality rate of 25% in patients needing mechanical ventilation in 1 large study.27 ECCO2R has been investigated in this population to see if it can help patients avoid needing mechanical ventilation.
Kluge et al,28 in a retrospective study, reported that 19 of 21 patients for whom noninvasive ventilation had failed were able to avoid intubation by receiving ECCO2R.
Burki et al,29 in a pilot study in 20 patients in 2013, found that a venovenous device provided clinically useful levels of carbon dioxide removal, significantly lowering the partial pressure of arterial carbon dioxide and raising the pH.
Del Sorbo et al30 found a lower risk for intubation when bilevel positive airway pressure and ECCO2R were used together instead of bilevel positive airway pressure alone. In addition, the hospital mortality rate was lower in the ECCO2R group.
The ECLAIR study31 (Extracorporeal Lung Assist to Avoid Intubation in Patients Failing NIV for Hypercapnic ARF) found that 14 of 25 patients with acute COPD exacerbation for whom noninvasive ventilation failed were able to avoid intubation with ECCO2R.
Azzi et al,32 in a 2021 study, similarly examined ECCO2R use in patients who were at risk of noninvasive ventilation failure; 85% of the patients (22 of 26) avoided intubation. Complications in this study included major bleeding in 7 (20%) of the ECCO2R recipients, which was, however, less than in earlier studies,31 despite a higher body mass index than in the control group (30 vs 25 kg/m2).
STATUS ASTHMATICUS
Asthma is a chronic inflammatory disorder of the airways, defined by variable and at least partially reversible airflow obstruction due to bronchial hyperresponsiveness to a variety of triggers.33 As in COPD, the natural course is marked by exacerbations with episodes of acute respiratory distress caused by increases in airway swelling, secretions, and muscle constriction. The most extreme form is called status asthmaticus. These exacerbations are the reason for many intensive care unit admissions, with many patients requiring intubation (61% in 1 series).34,35
Tiruvoipati et al36 used ECCO2R to treat 15 patients with acute or acute-on-chronic respiratory failure of various etiologies, including 2 with acute asthma exacerbation that required mechanical ventilation. In these 2 patients, the partial pressure of arterial carbon dioxide returned to near-normal levels within 6 hours, and both were discharged alive from the hospital.
Bromberger et al37 applied ECCO2R in 26 intubated patients with status asthmaticus, whose arterial blood pH and arterial partial pressure of carbon dioxide improved, allowing for lowering of inflation pressures on the ventilator. Additionally, the use of vasopressors was significantly decreased after ECCO2R initiation. Twenty patients were extubated while on ECCO2R, and all survived to hospital discharge.
While more research is required to see if ECCO2R can lower the mortality rate in this population, extracorporeal life support in asthma patients is associated with higher survival rates than in patients with other indications for extracorporeal life support,38 and we hope this benefit may extend to ECCO2R. ECCO2R is not currently approved for this status asthmaticus.
A BRIDGE TO LUNG TRANSPLANT
Many patients with end-stage lung disease experience an acute decline in respiratory status while waiting for a transplant.39,40 This can necessitate a bridging strategy with ECMO or ECCO2R. In such patients, “awake” ECMO (ie, with the patient awake, on ECMO, not on a ventilator) has been shown to have more favorable outcomes compared with mechanical ventilation.41 Given that ECCO2R has smaller cannula sizes, easier insertion techniques, and lower flow rates than ECMO, it may be a better method for awake bridging42 in those with primary hypercapnic respiratory failure, including those waiting for a repeat lung transplant.43
Benazzo et al44 reported on 120 patients bridged with extracorporeal life support from 1998 to 2017, of whom 26 received ECCO2R.
Fischer et al40 reported on arteriovenous ECCO2R in 12 patients with end-stage lung disease of various causes with ventilation-refractory severe hypercapnia and respiratory acidosis awaiting lung transplant. Ten patients underwent transplant, despite positive blood cultures in 7, use of mechanical ventilation, and need for extracorporeal life support, all of which are contraindications to lung transplant in some centers.
COMPLICATIONS
ECCO2R has been associated with various complications. The complex interplay between the patient’s sera and the artificial materials used in any extracorporeal device can lead to systemic inflammation by activating coagulation factors, platelets, leukocytes, and complement.3
Hemorrhage is common and can be catheter-related or at other sites such as the stomach, lungs, or brain. Doyle and Hunt45 estimated an incidence of severe hemorrhage of nearly 40% in patients on ECMO, and the use of systemic anticoagulation alone does not seem to be associated with bleeding risk.
Hemolysis has been reported in 2% to 11%,3 with post hoc analysis of the SUPERNOVA data noting higher rates of hemolysis and bleeding in patients on low-flow than on high-flow systems.18
Limb ischemia can occur in 4% to 10% but is encountered more with arterial than with venous catheterization,12 and it is of less concern in single-site venous cannulations.
Thrombocytopenia has been reported in patients on ECCO2R in rates ranging from 2% to 13%.18,20
DISCLOSURES
Dr. Lund has disclosed consulting for ALung Technologies. Tracy Dill has disclosed employment with ALung Technologies. Dr. Duggal has disclosed work as advisor or review panel participant and principal or co-investigator of funded research for ALung Technologies. The other authors report no relevant financial relationships which, in the context of their contributions, could be perceived as a potential conflict of interest.
- Copyright © 2022 The Cleveland Clinic Foundation. All Rights Reserved.