A 22-year-old medical student presented with dyspnea while walking to class. She reported slowly progressive dyspnea, fatigue, and reduced exercise tolerance over 6 months in addition to chills, night sweats, palpitations, and a weight loss of 5 pounds that she initially attributed to stress. She denied cough, chest pain, abdominal pain, nausea, and vomiting, and reported no changes in her menses or diet over several months.
Her medical history was significant for iron deficiency anemia owing to menorrhagia, which was resolved, and well-controlled gastroesophageal reflux disease. She confirmed compliance with her prescription medications of iron tablets and proton pump inhibitors and denied use of nonsteroidal anti-inflammatory drugs. She had undergone oral surgery in childhood, and her family history was significant for medulloblastoma in her brother, prostate cancer in her grandfather, and unknown cancer in her grandmother. Socially, she drank 2 alcoholic drinks on the weekends and denied smoking cigarettes or vaping. On examination, her vital signs included the following:
Heart rate 130 beats per minute
Temperature 39.1°C (102.4°F)
Oxygen saturation 94% on room air
Respiratory rate 18 breaths per minute
Blood pressure 128/68 mm Hg.
Significant physical examination findings were pallor, reduced breath sounds on the right hemithorax associated with dullness to percussion, and lack of egophony. She had palpable lymphadenopathy in her axillae bilaterally. The remaining clinical examination was unremarkable, with laboratory testing results as follows:
White blood cell count 12.0 × 109/L (reference range 4.5–11.0)
Hemoglobin 13.5 g/dL (12.1–15.1)
Platelet count 630 × 109/L (150–450)
Lactate dehydrogenase 279 IU/L (105–333)
C-reactive protein 275 mg/dL (< 10).
Liver enzymes, renal function, and coagulation studies were within reference ranges. A workup for infectious disease—including urinalysis, urinary antigens for pneumonia, blood cultures, respiratory culture, gram stain, and viral panel testing—was unremarkable.
IMAGING FINDINGS
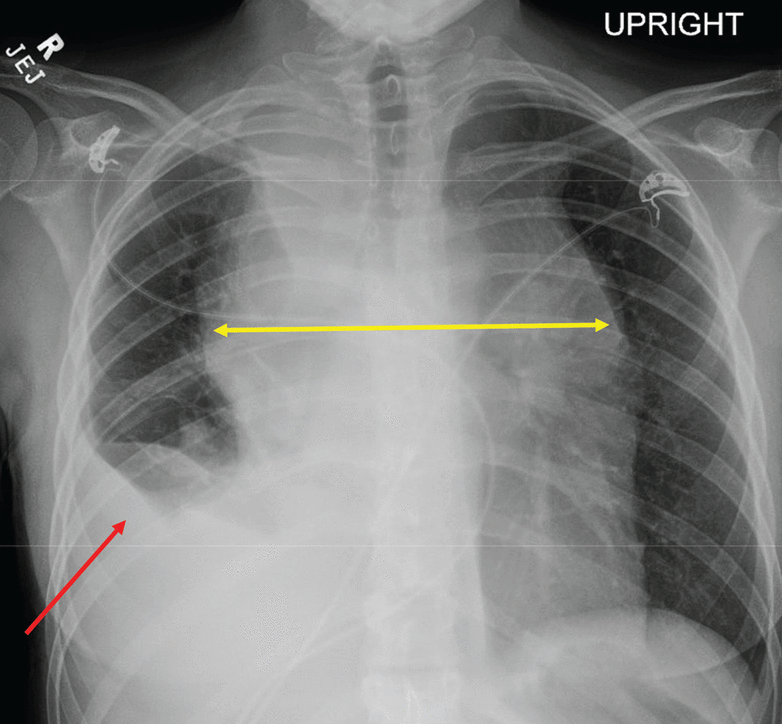
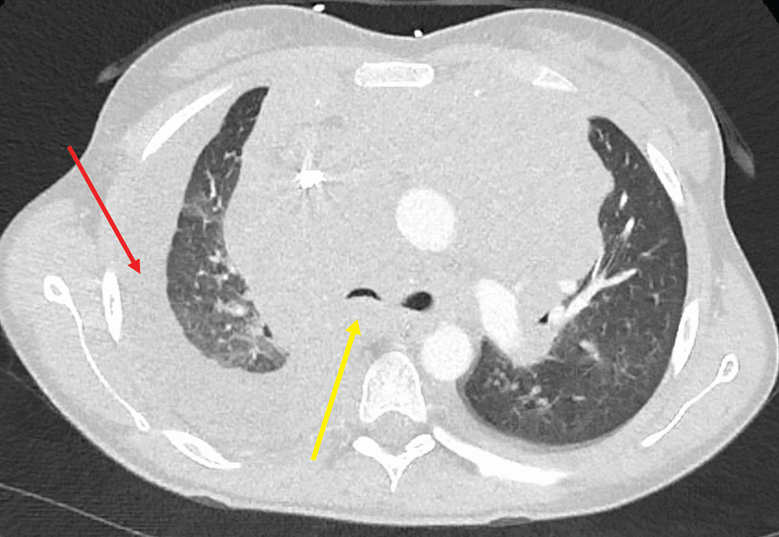
Her chest radiograph (Figure 1) was concerning for unilateral right pleural effusion in addition to a widened mediastinum. Lung fields were otherwise normal. Computed tomography of the chest (Figure 2) confirmed ill-defined bulky mediastinal and bilateral perihilar masses and bilateral axillary lymphadenopathy. The right mainstem and right distal bronchi were narrowed owing to extrinsic compression caused by the masses, with corresponding atelectasis of the right upper and middle lobes. A large right pleural effusion was seen. No filling defect was detected, ruling out pulmonary embolism. The lung parenchyma was otherwise normal without evidence of groundglass opacities, consolidation, nodules, cysts, or septal thickening.
Posterior-anterior chest radiography showed a widened mediastinum (yellow arrow) and right unilateral pleural effusion (red arrow).
Computed tomography of the chest with contrast showed atelectasis of the right upper and middle lobes secondary to extrinsic compression of the right mainstem by mediastinal lymphadenopathy (yellow arrow) and right unilateral pleural effusion (red arrow).
DIFFERENTIAL DIAGNOSES
1. What is the most likely cause of this patient’s unilateral pleural effusion?
Heart failure
Infection
Malignancy
Lymphangioleiomyomatosis
Hepatic hydrothorax
Chylothorax
The patient presented with a history of chronic dyspnea associated with mild weight loss and “B symptoms,” ie, night sweats, fever, and weight loss, suggesting lymphoma. She lacked chest pain, lower extremity edema, or paroxysmal nocturnal dyspnea and did not smoke cigarettes or indulge in excessive alcohol use. While having no personal risk factors for malignancy, she did have a significant family history.
The most likely cause of the unilateral pleural effusion in this patient would be malignancy owing to the slowly progressive dyspnea, B symptoms, and the presence of mediastinal masses. She lacked physical findings to suggest heart failure. The screen for infectious disease was negative, and imaging findings were inconsistent with infection or lymphangioleiomyomatosis. She did not have preceding trauma, chest surgery, history of congenital syndromes, or abnormal nails on her hands or feet to suggest chylothorax. Furthermore, liver enzyme tests were normal, making the presence of hepatic hydrothorax less likely. Table 1 summarizes key features of common causes of pleural effusions.1
Differential diagnosis of pleural effusion
Performing thoracentesis would be the most appropriate next step. This procedure would not only alleviate her dyspnea but also allow sampling of the pleural fluid for diagnostic purposes. Common laboratory tests performed on pleural effusion fluid are listed in Table 2.2–9 Dichotomization of pleural effusions into transudative or exudative subtypes utilizing the Light criteria8,10 is a key step in narrowing the differential diagnosis. Common causes of transudative effusions include heart failure, hepatic hydrothorax, nephrotic syndrome, or pulmonary embolism. Exudative pleural effusions are seen in infections, malignancies, autoimmune conditions, and pancreatitis to name a few. Light criteria analysis of pleural fluid has 99% sensitivity and 96% accuracy to identify exudative effusion.11
Pleural fluid analysis and rationale
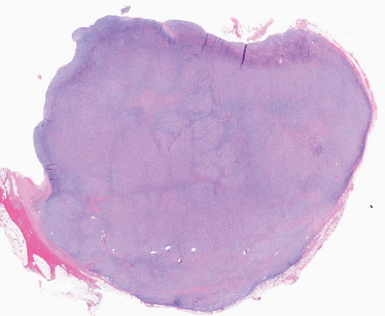
The patient underwent lung ultrasonography to assess the safety of performing thoracentesis and secondarily to determine the site of needle insertion. Thoracentesis resulted in the drainage of 1,500 mL of green-hued, thin, nonviscous pleural fluid (Figure 3). It was lymphocytic and exudative by the Light criteria. Bilirubin and triglyceride analysis of the pleural fluid was done due to its green color. It revealed a bilirubin count of 0.6 mg/dL, a pleural fluid-serum bilirubin ratio of 1, and a triglyceride of 30 mg/dL. Culture and Gram stain were negative. Cytopathology was concerning for the presence of atypical lymphocytes. Given the presence of widespread lymphadenopathy on imaging, there was concern for hematopoietic malignancy.
Green pleural fluid.
2. What was the cause of green pleural effusion?
Biliothorax
Chylothorax
Empyema
Malignancy
The most common cause of green pleural fluid is biliothorax.12,13 Most often seen following trauma or surgery, the green color is a consequence of biliary leakage intraperitoneally. Biliary fluid may seep into the pleural space via diaphragmatic pores, leading to the accumulation and development of pleural effusion. The exact mechanism of green pleural fluid in other diseases such as empyema, chylothorax, and malignancy is less well understood. It has been theorized that green pigments are produced by bacteria in empyema.14 Some authors have suggested that an increase in pleural fluid viscosity owing to excess pleural fluid protein and cellularity may cause the green color in chylothorax and malignant pleural effusions.15–18
In our patient, the pleural fluid-serum bilirubin ratio of 1, along with a low pleural fluid bilirubin level, ruled out biliothorax.19,20 Additionally, she had no recent abdominal surgeries or trauma to cause biliary leak. As her triglyceride level was less than 110 mg/dL, chylothorax was unlikely. Culture data from pleural fluid analysis were unrevealing. Malignancy is the most likely cause of green pleural effusion in this patient as it was exudative and lymphocytic in nature.
3. What are the next steps in view of the lymphadenopathy and abnormal cytopathology of the pleural fluid?
Repeat thoracentesis for serial cytopathologic assessment
Pleural biopsy
Fine-needle aspiration
Core needle biopsy
Excisional lymph node biopsy
The diagnostic yield for malignancy on pleural fluid cytopathology is low, between 40% and 60%.21,22 Additional sampling of pleural fluid increases sensitivity by 27%, beyond which there is no improvement in the diagnostic yield with subsequent procedures.23,24 The type of malignancy also influences the diagnostic yield of cytopathologic analysis of pleural fluid.21 Generally, the highest yield is for adenocarcinomas—including lung, ovarian, breast, and pancreatic—and is much lower for head and neck cancers, sarcomas, renal, and lymphomas.21,22,25 For hematopoietic malignancies like lymphoma, the diagnostic yield of pleural fluid cytology is between 22.2% and 94.1%.22,25,26 Thus, repeating thoracentesis would not be the appropriate next step in this case.
Blind pleural biopsy is commonly used worldwide, specifically in the diagnostic algorithm for tuberculous pleural effusion. The diagnostic yield of pleural biopsy for malignancy is low, akin to that of pleural fluid analysis.23,27 Imaging-assisted thoracoscopic pleural biopsy has a much higher diagnostic yield for malignancy28 but it is not routinely performed owing to cost and the requirement for a specially trained surgeon or pulmonologist.1 Although a cohort study of 34 patients reported a superior diagnostic yield of pleural biopsy compared with pleural fluid analysis for the diagnosis of lymphoma,29 pleural involvement in lymphoma is usually part of a systemic manifestation and is only rarely the primary source.
The gold standard technique for diagnosing malignant lymphomas is excisional lymph node biopsy.30 The lymph node is removed in its entirety by a surgeon to allow for complete histologic examination of the lymph node architecture. Based on the findings, provisions for other tests such as flow cytometry and molecular genetic testing can be made.30,31
Some criticisms of excisional lymph node biopsy relate to its invasive nature and higher rate of bleeding and infection. However, if the lymph nodes are superficial, it is an excellent diagnostic test. Core needle biopsy and fine-needle aspiration are minimally invasive procedures. Core needle biopsy uses a wider needle than fine-needle aspiration, thus a larger tissue sample may be obtained.32,33 Core needle biopsy has a much higher diagnostic yield than fine-needle aspiration and is more common.34 Studies have demonstrated that fine-needle aspiration has a diagnostic yield as low as 29%, and only 2% when correlated with findings of excisional lymph node biopsy.35,36 Thus, fine-needle aspiration overall has a low yield and is inferior to core needle biopsy and excisional lymph node biopsy.36 Even though core needle biopsy is gaining popularity, a recent large-scale study of lymphoma patients found excisional lymph node biopsy to have superior diagnostic yield compared with core needle biopsy. There was an increased risk of a nondefinite diagnosis with core needle biopsy.37 In this case, the presence of multiple superficial axillary lymph nodes—coupled with a patient with a low-risk profile to undergo general anesthesia—argues for the use of excisional lymph node biopsy as the most appropriate diagnostic test.
In our patient, pathologic analysis of the excisional lymph node biopsy confirmed the diagnosis of lymphocyte-rich Hodgkin lymphoma. Figure 4 illustrates the complete distortion of the usual lymph node architecture owing to the presence of atypical polymorphous infiltrates. Hematoxylin and eosin staining (Figure 5) revealed numerous large and atypical lymphocytes characterized by large-sized, irregular, and multilobed nuclei. The nucleoli ranged from single to multiple. Some mummified cells were identified, with condensed cytoplasm and pyknotic eosinophilic or basophilic nuclei. The cells were morphologically compatible with Hodgkin and Reed-Sternberg cell morphology. By immunohistochemistry, the cells of interest were positive for PAX5 (weak), CD30, and CD15 stains, and negative for CD3, CD20, CD45, CD43, CD2, and LK1. Flow cytometry showed no phenotypic evidence of non-Hodgkin lymphoma.
Pictograph of the whole lymph node illustrating the complete effacement of the normal lymph node architecture by atypical polymorphous infiltrates.
The characteristic Reed-Sternberg cell (red arrow) on excisional lymph node biopsy study (hematoxylin and eosin, magnification × 40).
STAGING
To determine the stage of classic Hodgkin lymphoma, our patient underwent positron emission tomography, which revealed mediastinal, pericardial, hilar, axillary, and subpectoral lymphadenopathy with extension of hypermetabolic activity into the central spinal canal at level T5 of the spine. Thus, she was diagnosed with stage IV classic Hodgkin lymphoma owing to the involvement of multiregional lymphoid tissue above and below the diaphragm, in addition to extranodal tissue of the axial spine.
TREATMENT
A chemotherapy regimen of doxorubicin, vinblastine, dacarbazine, and brentuximab was initiated. The patient lacked neurologic symptoms and signs to suggest spinal stenosis or cauda equina syndrome. Thus, the T5 lesion was monitored without the initiation of steroids or radiation therapy. Her dyspnea had improved following thoracentesis, but she experienced intermittent central chest pain, which was attributed to the mediastinal mass effect. After the initiation of chemotherapy, she reported the resolution of her symptoms. She was discharged home with outpatient oncology follow-up.
PLEURAL EFFUSIONS IN HODGKIN LYMPHOMA
Pleural effusions are seen at presentation in patients with lower stages of Hodgkin lymphoma, with rates ranging from 10% to 30%.38,39 When present, these effusions are usually associated with poor survival, warranting the initiation of aggressive up-front therapy.38,39 The pleural effusions in Hodgkin lymphoma are typically exudative, lymphocytic, and serosanguinous in nature.40 A jelly-like consistency of pleural fluid is associated with mesothelioma.41 Chylothorax has often been associated with lymphoma owing to the obstruction of the lymphatic vessels or the thoracic duct by the tumor or bulky lymphadenopathy.40,42 Chylothoraces are usually white or milky in appearance. The color of effusions in Hodgkin lymphoma can vary from serous to bloody. The color of the pleural fluid has no correlation with prognosis.43 Black pleural fluids have been reported in malignancy and chronic hemothorax owing to the abundance of hemosiderin-laden macrophages.
Our patient’s case illustrates a novel presentation of green-colored unilateral pleural effusion associated with classic Hodgkin lymphoma. A comprehensive history and physical examination allow the clinician to narrow the differential diagnoses in the hopes of obtaining high-yield tests to shorten the time it takes to arrive at the true diagnosis and expedite treatment. Laboratory analysis of pleural fluid and its characterization by color and viscosity play crucial parts in the diagnostic algorithm for the etiology of pleural effusions. As illustrated in this case, a systematic and algorithmic approach to the management of pleural effusion is vital.
TAKE-HOME POINTS
New-onset pleural effusion must be evaluated by analysis of the pleural fluid for diagnostic purposes.
Prior to thoracentesis, an adequate amount of pleural fluid must be present. The safety of the procedure can be confirmed with lung ultrasonography.
Thoracentesis may function as a therapeutic tool to alleviate dyspnea.
Meticulous visual inspection to characterize the color and viscosity, in addition to laboratory analysis of pleural fluid including the utilization of the Light criteria, is paramount to determine the etiology of the pleural effusion as it may necessitate additional testing of the pleural fluid.
Excisional lymph node biopsy is the gold standard test for the diagnosis of lymphoma.
DISCLOSURES
The authors report no relevant financial relationships which, in the context of their contributions, could be perceived as a potential conflict of interest.
Acknowledgment
We thank Carmen Fullmer, MS, for her assistance and support in preparing this manuscript.
- Copyright © 2023 The Cleveland Clinic Foundation. All Rights Reserved.