ABSTRACT
Many patients with chronic kidney disease have chronically elevated cardiac troponin levels, and if they present with symptoms suggesting an acute coronary syndrome, it is often difficult to determine if this is the correct diagnosis. This article briefly reviews the major challenges in diagnosing acute coronary syndrome in patients with chronic kidney disease, describes the mechanisms and prognostic significance of troponin elevation in chronic kidney disease, and provides a diagnostic algorithm to risk-stratify patients with chronic kidney disease who have troponin elevation and suspected acute coronary syndrome.
Clinicians should use troponin measurements judiciously within a suggestive clinical context and, even more than in the general population, rely on serial testing in light of the high prevalence of elevated baseline troponin levels in patients with chronic kidney disease, even in the absence of acute coronary syndrome.
Because troponin assays vary in diagnostic accuracy, clinicians should be familiar with the characteristics of the local assay used at their institution.
In addition to troponin levels, clinicians should integrate presenting symptoms, traditional risk factors, electrocardiographic changes, and echocardiographic findings.
Troponin elevations pose a dilemma in patients with chronic kidney disease. Cardio vascular disease accounts for about half of deaths in patients with chronic kidney disease, making it the leading cause of death in this high-risk group.1,2 Acute coronary syndrome is diagnosed on the basis of a combination of symptoms, changes on electrocardiography, and a typical rise and fall in cardiac troponin I and cardiac troponin T levels. An increase in cardiac troponin beyond the 99th percentile (the upper reference limit) is clinically relevant, and peak troponin levels inform a risk-based strategy. However, the high prevalence of chronically elevated cardiac troponin in chronic kidney disease makes a biomarker-based diagnosis of acute coronary syndrome challenging.
No validated cutoff values of troponin specific to patients with chronic kidney disease are available. Hence, isolated measurements are difficult to interpret, especially when baseline levels are not known on presentation. Any acute kidney injury in which the serum creatinine level increases by 40% to 60% may result in a 20% rise in cardiac troponin, followed by a subsequent fall during the recovery phase of the kidney injury, mimicking the pattern seen in acute coronary syndrome.3
The influence of dialysis on troponin levels remains controversial due to paucity of data on the confounding effects of sample timing and type of dialysis.4 A small observational study of patients without known heart disease who were about to begin dialysis found that those with asymptomatic multivessel coronary artery disease already had higher cardiac troponin levels.5
Adding to the diagnostic uncertainty, the presenting symptoms of acute coronary syndrome are more commonly atypical (eg, isolated dyspnea) in patients with chronic kidney disease,6 and are often misattributed to nonischemic phenomena such as interdialytic volume overload or intradialytic fluid shifts. When patients with chronic kidney disease do experience an acute coronary syndrome, the prevalence of chest pain (the typical pathognomonic symptom) is inversely related to the patient’s stage of chronic kidney disease, as low as 40% in those with advanced disease.7
In addition, many patients with chronic kidney disease have abnormal ST-T patterns on electrocardiography even in the absence of acute coronary syndrome, and these may be due to electrolyte abnormalities, left ventricular hypertrophy, and conduction abnormalities, making acute coronary syndrome even more difficult to recognize.4
INCREASED RELEASE AND DECREASED CLEARANCE
Troponin levels can be elevated in chronic kidney disease as a result of increased release by injured myocytes, decreased clearance by the failing kidneys, or both.
Emphasis was traditionally placed on decreased clearance, as troponin fragments that would normally be excreted by the kidneys would accumulate in patients with decreased glomerular filtration.8 However, numerous factors specific to chronic kidney disease contribute to chronic myocardial injury, resulting in an ongoing source of cardiac troponin. These include coronary microvascular disease, left ventricular hypertrophy, hypoperfusion related to hypotension or anemia, and cardiotoxic uremic toxins.8
Notably, regenerating skeletal muscle reverts back to expressing embryonic forms of cardiac troponin T, but not cardiac troponin I, in response to an increased catabolic state in chronic kidney disease.9
TROPONIN IS AN IMPORTANT PROGNOSTIC FACTOR
The Acute Catheterization and Urgent Intervention Triage Strategy trial randomized 13,819 patients at moderate and high risk with non-ST-elevation myocardial infarction to different antithrombotic regimens and an early invasive strategy.10 Of these patients, 2,179 had established chronic kidney disease, and in this group, those with cardiac troponin elevation had higher rates of death or myocardial infarction at 30 days (hazard ratio 2.05) and at 1 year (hazard ratio 1.72). However, the absolute level of baseline elevation did not add further prognostic precision, likely because of the numerous confounding factors specific to chronic kidney disease.10
In a large multicenter prospective trial in patients with low estimated glomerular filtration rates and suspected acute coronary syndrome, troponin I levels higher than the 99th percentile (in this study, 16 ng/L in women or 34 ng/L in men, measured by high-sensitivity assay) accurately identified those at high risk for acute coronary syndrome or cardiac death within 1 year, whereas patients with levels lower than 5 ng/L were at low risk.11
STEPWISE APPROACH TO THE DIAGNOSIS
Physicians should measure cardiac troponin levels within the appropriate clinical context and, even more so in patients with chronic kidney disease, commit to serial testing, given the low positive predictive value of individual samples if the baseline level is unknown. The following are important considerations.
Follow serial cardiac troponin levels
For patients with chronic kidney disease and signs that suggest acute coronary syndrome, we recommend tracking the rise and fall of cardiac troponin levels over the 3 hours after presentation with high-sensitivity assays, or over 6 hours with conventional assays, or up to 9 hours in those with end-stage renal disease, rather than documenting a single value (or a rapid change over a 1-hour period), which is in line with national guidelines.12,13
Gunsolus et al14 found that serial troponin I measurements over 3 to 6 hours, using a 99th percentile cutoff (16 ng/L in women and 34 ng/L in men), had a sensitivity for myocardial infarction of at least 95% in patients presenting to the emergency department with symptoms suggesting ischemia, across the spectrum of chronic kidney disease, including dialysis patients. However, the specificity decreased with decreasing renal function.
Baseline levels may help contextualize new elevations in the absence of significant kidney function changes. Patient characteristics (eg, sex) can affect high-sensitivity troponin values. However, no prospective trials of longitudinal cardiac troponin assessment have been done to justify baseline testing in patients with chronic kidney disease, especially given the lack of clear guidelines on the need for further testing in the presence of asymptomatic troponin elevations.
Troponin assays are not all the same
High-sensitivity troponin assays should be preferred because they have shown greater accuracy, especially when applied serially, given their lower coefficient of variation.14 However, high-sensitivity troponin assays are not all the same (Table 1).4,15–21 Even assays from the same manufacturer may not be interchangeable because manufacturers continually make changes to antibodies and calibration materials. Therefore, it is imperative that clinicians familiarize themselves with their institution’s current assay.
Troponin cutoffs for acute coronary syndrome in patients with chronic kidney disease
Although guidelines do not specify a preferred assay, troponin I has been observed to be less affected than troponin T by renal dysfunction and more specific for myocardial injury in patients with chronic kidney disease.11,22 In fact, experimental data suggest that cross-reactivity with fetal cardiac troponin T isoforms re-expressed in diseased or regenerating skeletal muscle may contribute to elevated cardiac troponin T levels in patients with chronic kidney disease without symptoms.23 In contrast, cardiac troponin I has never been found to be expressed in skeletal muscle at any point during development or in adverse muscular dystrophy.23–25
Threshold for diagnosis
For patients with chronic kidney disease whose baseline troponin levels are already above the 99th percentile, a 20% rise on serial measurements is indicative of an ongoing myocardial injury and would be a reasonable threshold change for a suspected diagnosis of acute coronary syndrome.13
When initial levels are only mildly elevated (eg, high-sensitivity troponin T < 20 ng/L), any absolute change greater than 5 or 10 ng/L should raise concern for acute coronary syndrome. In fact, several studies have reported small absolute changes in troponin in patients with acute coronary syndrome,26–30 possibly as a result of plaque rupture occurring days before clinical presentation, when troponin samples are taken during the plateau phase of troponin release. Therefore, symptom duration should inform the significance of small troponin changes in patients with chronic kidney disease with suspected acute coronary syndrome.
Chronic kidney disease vs acute kidney injury
Kidney injury, defined as an estimated glomerular filtration rate less than 60 mL/min/1.73 m2 or an albumin-creatinine ratio 3 mg/mmol or higher, should be considered chronic only if it has been present for at least 3 months before presentation. Acute kidney injury in the setting of acute coronary syndrome is relatively common and has different diagnostic consequences compared with stable chronic kidney disease.
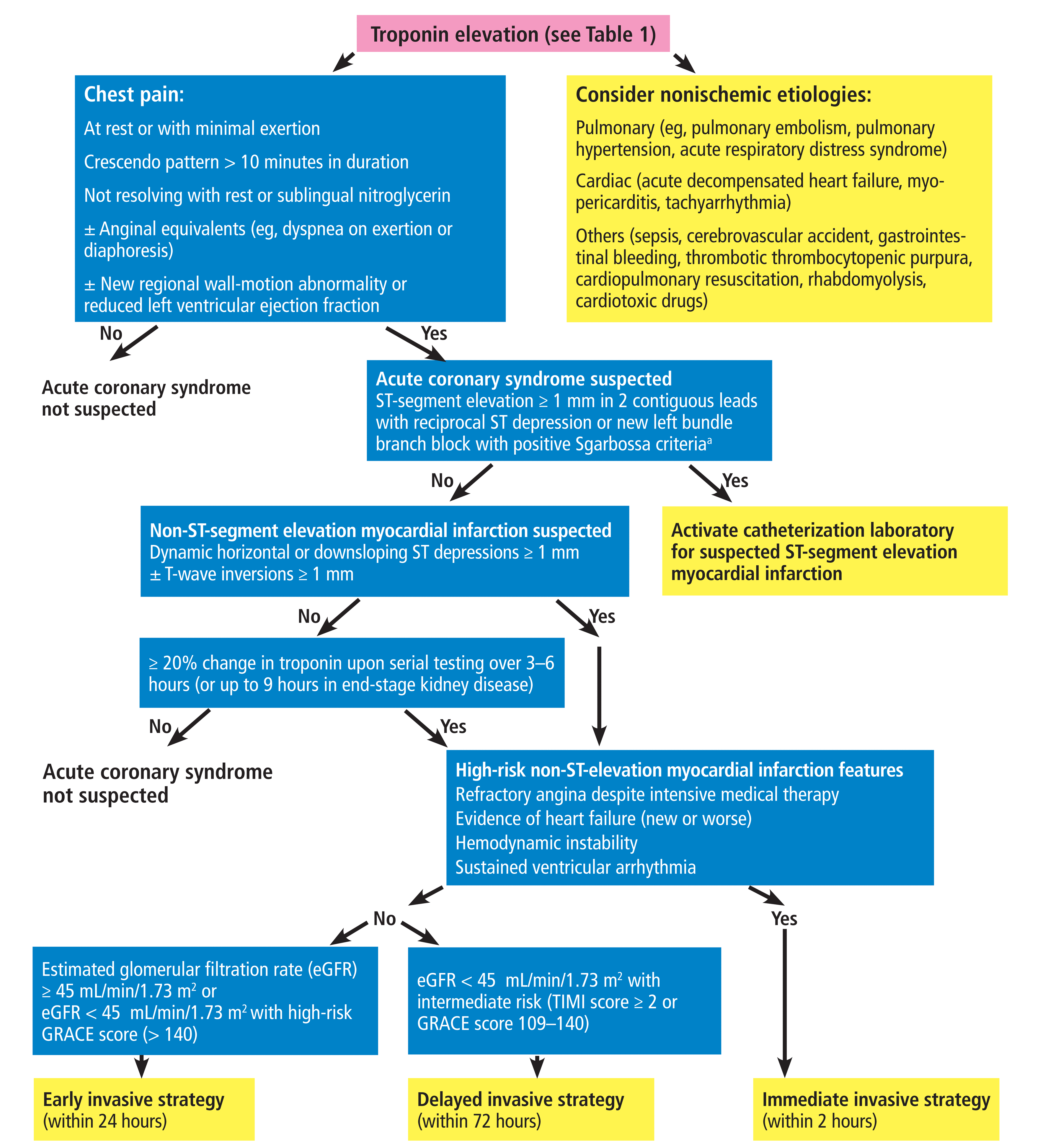
A proposed workup algorithm
A workup algorithm is shown in Figure 1. Briefly, one should use clinical judgment, integrating coronary artery disease risk factors, high-risk symptoms, typical electrocardiographic changes, and echocardiographic findings, which affect the predictive value of cardiac troponin testing and help to determine the likely mechanism of myocardial injury. The numerous possible nonischemic causes of cardiac troponin elevation should be entertained early in the differential diagnosis.
Diagnostic evaluation of elevated troponin in patients with chronic kidney disease and suspected acute coronary syndrome.
aSgarbossa criteria: Das D, McGrath BM. Sgarbossa criteria for acute myocardial infarction. CMAJ 2016; 188(15):E395. doi:10.1503/cmaj.150195. GRACE = Global Registry of Acute Coronary Events; TIMI = Thrombolysis in Myocardial Infarction
Regardless of the frequent atypical presenting symptoms often misattributed to noncardiac causes, a newly reduced ejection fraction accompanied by regional wall-motion abnormalities is highly suspicious for acute coronary syndrome.
New ST-segment elevation
Electrocardiographic evidence of new ST-segment elevation together with a compelling acute coronary syndrome presentation should prompt catheterization laboratory activation irrespective of the troponin level. Broad, asymmetrically peaked (“hyperacute”) T waves or loss of precordial T-wave balance may be seen in the early stages of ST-segment elevation myocardial infarction (STEMI). Also, keep in mind other common STEMI equivalents (eg, biphasic T waves)31 and the possibility that coronary artery occlusion may fail to result in electrocardiographic findings that meet STEMI criteria (ie, subtle STEMI). In the latter case, a formula based on 4 electrocardiographic variables has been proposed to assist clinicians.32
In contrast, careful comparison of electrocardiographic patterns and troponin levels with earlier baseline values (or serial monitoring, if those are unavailable) would help identify patients with non-ST-segment elevation myocardial infarction (NSTEMI). Current guidelines advocate for an early invasive strategy in unselected patients with high-risk NSTEMI (eg, hemodynamic or electrical instability), but patients with moderate and severe chronic kidney disease were underrepresented in landmark trials. This is important, since delaying angiography may be desirable for 2 reasons in highly selected patients with chronic kidney disease with NSTEMI. First, optimal medical therapy may allow the plaque to stabilize and mitigate the thrombus burden. Second, it would enable preprocedural optimization of hemodynamically unstable patients, administration of intravenous fluid to minimize postprocedural acute kidney injury, or both.
A 2009 analysis of 23,262 patients with chronic kidney disease with NSTEMI in the Swedish Web System for Enhancement and Development of Evidence-Based Care in Heart Disease Evaluated According to Recommended Therapies registry33 showed a 1-year survival advantage in patients with mild to moderate chronic kidney disease undergoing an early invasive strategy, but no apparent benefit among those with severe chronic kidney disease. The theoretical benefits of an early invasive strategy were possibly outweighed by the higher procedural risks in patients with stage 4 or 5 chronic kidney disease. Notably, the outdated definition of early invasive strategy (ie, angiography and possible revascularization within 14 days of admission, whereas now it means within 24 hours of admission) and the use of old-generation stents would limit its generalizability to current practice.
Along the same lines, a propensity-matched analysis published in 202234 found no significant reduction in mortality rates with an early invasive strategy in patients with an estimated glomerular filtration rate less than 45 mL/min/1.73 m2.
Lacking randomized studies specifically in patients with chronic kidney disease, the available data would justify an immediate invasive strategy (ie, within 2 hours) only in patients with chronic kidney disease with high-risk NSTEMI features (eg, hemodynamic or electrical instability), and an early invasive strategy (ie, within 24 hours) in patients with NSTEMI and chronic kidney disease stage 2 or 3a (Figure 1).
Conventional risk scores—eg, GRACE (Global Registry of Acute Coronary Events) or TIMI (Thrombolysis in Myocardial Infarction) score—should guide the need for an early invasive strategy in non-high-risk NSTEMI patients with chronic kidney disease stages 3b through 5, who would otherwise be at higher than average risk for periprocedural complications. While the change in troponin level is critical in diagnosing acute coronary syndrome, initial abnormal biomarker status recorded at presentation should be used for prognostication with the GRACE or TIMI score.35
DISCLOSURES
The authors report no relevant financial relationships which, in the context of their contributions, could be perceived as a potential conflict of interest.
- Copyright © 2023 The Cleveland Clinic Foundation. All Rights Reserved.