A 44-year-old man presented to an outpatient clinic after 11 days of fever, chills, headache, and nausea. He was a coffee roaster by trade, and his symptoms had started about 10 days after returning from a 3-week trip to buy coffee in Ethiopia. He said his fever would come and go, and the last episode was 2 days earlier. He denied any diarrhea, constipation, rash, or lymphadenopathy.
The patient appeared lethargic. Examination of his heart, lungs, and abdomen was unremarkable. His vital signs were:
Temperature 38.9°C (102.0°F)
Heart rate 80 beats per minute
Respiratory rate 14 breaths per minute
Blood pressure 142/80 mm Hg
Oxygen saturation 97% on room air.
He had been treated for malaria in Tanzania when he fell sick there a few years earlier. He said he took chloroquine to prevent malaria every time he went abroad, as directed for his earlier trips. He had received the yellow fever virus vaccine because of his frequent travel to the tropics and was up-to-date on his routine childhood and pretravel immunizations. On his last trip, he had not been exposed to local domestic or wild animals, had not had any sexual encounters, had not drunk any unclean water, and had not eaten any raw or improperly cooked food.
DIFFERENTIAL DIAGNOSIS OF FEVER IN A RETURNING TRAVELER
1. What is the most likely cause of this patient’s fever?
Malaria
Typhoid fever
Influenza
Yellow fever
Meningococcemia
Measles
The differential diagnosis for fever with a medium to long incubation period in a returning traveler is broad. Providers should consider the infections endemic to the region where the patient traveled (wwwnc.cdc.gov/travel).
Thwaites and Day1 proposed a risk-based approach using the Quick Sepsis-Related Organ Failure Assessment (qSOFA) score, signs of severe disease (cyanosis, meningism, peritonism, digital gangrene), and possibility of a highly transmissible infection (eg, Middle East respiratory syndrome-coronavirus [MERS-CoV], Ebola) as an initial assessment to identify and treat life-threatening causes of fever. A detailed history of exposure to unclean water, animals, insects, bites, or raw or improperly cooked food is crucial in building a robust differential diagnosis.2
Malaria
Fever in a traveler returning from an area where malaria is endemic (see www.cdc.gov/malaria/travelers/country_table/) is an emergency. Major clinical features of malaria are fever (present in 92% of cases in 1 study), chills (78%), headache (64%), and nausea and vomiting (35%)—and our patient had all of these. Other possible symptoms such as myalgia (53%) and diarrhea (26%) are sometimes mistaken for symptoms of influenza or infectious gastroenteritis.3
In another study,4 Plasmodium falciparum malaria was the most common cause of fever in US residents returning from sub-Saharan Africa (accounting for 12.78% of cases), followed by acute unspecified diarrhea (9%), acute bacterial diarrhea (5.59%), and giardiasis (4.23%).
Malaria is transmitted by the bite of a female Anopheles mosquito.5 Most Anopheles mosquitoes are not exclusively anthropophilic (preferring to feed on humans). However, the primary malaria vectors, A gambiae and A funestus, are strongly anthropophilic and are the two most efficient malaria vectors worldwide.
Our patient’s symptoms were consistent with malaria. Moreover, although he was taking malaria chemoprophylaxis, he was not taking the right one, as there is a high incidence of chloroquine-resistant P falciparum malaria in Africa. The prolonged incubation period also points to malaria (Table 1).
Incubation periods of common travel-related infectionsa
Finally, although our patient’s pulse rate of 80 beats per minute seems normal, it is actually lower than expected, given his fever. Assessing vital signs for relative bradycardia is a great tool to discern several medical conditions, and malaria is one of the causes (Table 2). However, the most common cause of relative bradycardia is the use of beta-blockers.6,7
Causes of relative bradycardia
Typhoid fever
Typhoid fever, caused by Salmonella typhi, is a common cause of travel-related fever. In 2002, an estimated 408,837 cases of typhoid fever occurred in Africa.8 However, precise numbers are not available, since many hospitals in Africa do not have laboratories capable of performing the blood cultures essential for the diagnosis of typhoid fever. In addition, typhoid fever is often mistaken for malaria.
Typhoid fever has an incubation period of about 1 week, which makes it less likely to be the cause of this patient’s illness. However, in rare cases, the incubation period can be as long as 3 weeks.9
The patient said he had no diarrhea or constipation, which also makes typhoid fever less likely. Moreover, typhoid fever is more commonly associated with high unremitting fever, which is inconsistent with the patient’s fever pattern.
Influenza
Influenza is uncommon in warm-weather months; however, the seasons are reversed in the Southern and Northern hemispheres. Also, physicians should suspect influenza at any time of year in travelers returning from the tropics, where influenza can occur year-round.10 However, the incubation period of influenza is typically 1 to 4 days, which was inconsistent with our patient’s history.
Yellow fever
Yellow fever should be suspected if an unvaccinated traveler returns from sub-Saharan Africa or forested areas of Amazonia with fever, jaundice, hemorrhage, and renal failure.
The mosquito vectors of yellow fever are Aedes species in Africa and Haemogogus species in South America. Aedes mosquitoes are also vectors for dengue virus (symptoms: high fever, sudden-onset skin rash, myalgia, headache, and mild hemorrhagic manifestations), West Nile virus, Chikungunya (symptoms: high fever, headache, myalgia, and moderate to severe arthralgia), eastern equine encephalitis virus, and Zika virus (symptoms: low-grade fever, descending rash, myalgia, conjunctivitis, headache, edema, and vomiting) (Table 3).11
Diseases that mosquitoes carry
Our patient had relative bradycardia, which can be seen in yellow fever. However, the incubation period for yellow fever is short, 3 to 6 days (median 4.3 days) after the bite of an infected mosquito.12 Moreover, he had been vaccinated against yellow fever.
Meningococcemia
Meningococcemia, caused by Neisseria meningitides serogroups A, B, C, W, X, and Y, is a life-threatening illness if not treated promptly. Travelers returning from the “meningitis belt” of sub-Saharan Africa who have symptoms consistent with this diagnosis should be suspected of having it, especially during the dry season (December–June). Symptoms generally surface 1 to 10 days after exposure (which is a short incubation period) and present as meningitis half of the time. The clinical manifestations include sudden onset of headache, fever, neck stiffness, and petechial or purpuric rash, which did not fit our patient’s presentation.
Measles
Measles is considered the most contagious viral disease known, and its incidence in Ethiopia is high, with 49 cases per million population in 2016.13 The incubation period ranges from 7 to 21 days from exposure to onset of fever. A clinical diagnosis of measles can be made from the clinical features of generalized maculopapular rash lasting for 3 or more days, temperature of 38.3°C (100.9°F) or higher, and cough, coryza, and conjunctivitis.
These clinical features did not fit our patient’s presentation; moreover, he had been vaccinated against measles.
All of the infections discussed above can be prevented with appropriate pretravel vaccinations and chemoprophylaxis.
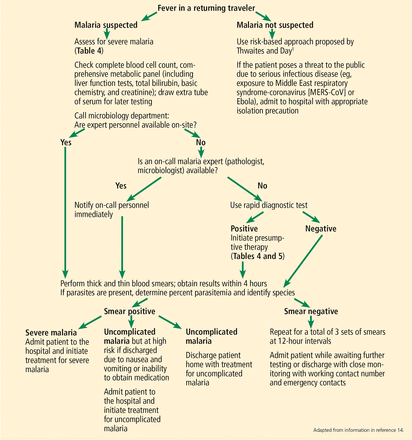
DIAGNOSTIC TESTING FOR MALARIA
2. If a pathologist or microbiologist is not available on call, how is the diagnosis of malaria made?
Blood culture
Plasmodium species polymerase chain reaction (PCR)
Plasmodium species rapid diagnostic test, then thick and thin blood films when an expert is available to look at them
Plasmodium serologic study
The best choice in this situation is Plasmodium species rapid diagnostic test, followed by thick and thin blood films.
Light microscopy is the gold standard
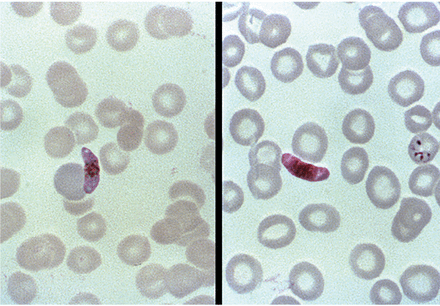
Light microscopy of blood smears with Giemsa staining (to give parasites a distinctive appearance) remains the gold standard for malaria diagnosis if qualified staff are available to do it immediately (Figure 1). The thick film is used to screen for parasites using hypotonic saline to lyse red blood cells. The thin film is then used to identify the species of Plasmodium. Blood films should be prepared and read immediately by experienced personnel.
Workup of fever in a returning traveler.
Rapid diagnostic tests
If expert personnel are not readily available to examine a blood smear, a rapid diagnostic test should be performed immediately (Figure 2).14
Two Giemsa-stained, thin-film blood smear photomicrographs. Left, a Plasmodium falciparum macrogametocyte; right, a microgametocyte. Image by US Centers for Disease Control and Prevention, Steven Glenn, Laboratory & Consultation Division 1979.
There are two types of rapid diagnostic tests for malaria. The first is based on detection of Plasmodium histidine-rich protein-2 (HRP-2), which is closely associated with the development and proliferation of the parasite. The only test of this type approved and available in the United States is BinaxNOW Malaria (www.alere.com/en/home/product-details/binaxnow-malaria.html), which has a reported sensitivity of 96% and specificity of 99% for Plasmodium infection compared with microscopy.15 This test is approved for use by hospital and commercial laboratories, not by individual clinicians or by patients themselves.
However, HRP-2 tests have limitations. Common causes of false-negative results include:
P falciparum strains that do not express HRP-2
Nonfalciparum species (P vivax, P ovale, P malariae, P knowlesi)
Low-level parasitemia (100–1,000/μL).
The second type of rapid diagnostic test, which is not available in the United States, is based on detection of P falciparum-specific lactate dehydrogenase and pan-Plasmodium lactate dehydrogenase. It has a sensitivity of 80% and a specificity of 98% for Plasmodium infection compared with microscopy.15
Rapid diagnostic tests take only 2 to 15 minutes and are highly specific; hence, a positive result should prompt immediate treatment. However, a negative result still requires a blood smear to detect low-level parasitemia or nonfalciparum species. Therefore, regardless of the rapid diagnostic test result, microscopy must always be performed afterward (Figure 2).14
Polymerase chain reaction
Although PCR testing for Plasmodium is available in commercial laboratories, the turn-around time may be unfavorable when an immediate medical decision is needed. It can, however, be beneficial in identifying the Plasmodium species (eg, P vivax and P ovale), which may further guide the need for presumptive antirelapse therapy (previously known as terminal prophylaxis).
Serologic testing
Serologic Plasmodium testing only assesses past exposure and has no utility in the acute setting.
Blood culture
Malaria diagnosis cannot be established through blood culture. Hence, that is not the correct answer to the question. However, if a provider suspects a bacterial coinfection with bacteremia (eg, Salmonella species or Escherichia coli), obtaining blood culture should be considered. In a small study of 67 adults hospitalized for P falciparum, 13% (95% CI 5.3%–21.6%) were bacteremic on admission.16
CASE CONTINUED: LABORATORY RESULTS
A rapid diagnostic test was ordered for our patient and was positive for P falciparum. On-call expert personnel were available to read the blood film. The level of parasitemia was 4% of red blood cells infected. Results of other blood tests were as follows:
Hemoglobin 10 g/dL (reference range 13.0–17.0)
White blood cell count 15.0 × 109/L (3.70–11.00)
Platelet count 150 × 109/L (150–400)
Glucose 60 mg/dL (65–100)
Carbon dioxide 20 mmol/L (23–32)
Creatinine 1.5 mg/dL (0.70–1.40)
Total bilirubin 1.2 mg/dL (0.2–1.0).
The patient was immediately transferred to the emergency department to be treated and monitored.
TREATMENT OF MALARIA
3. What treatment should this patient receive?
Chloroquine phosphate
Hydroxychloroquine
Primaquine
Atovaquone-proguanil
Our patient appeared to have uncomplicated P falciparum infection from a chloroquine-resistant region. A patient who presents with symptoms of malaria and a positive malaria test without features of severe malaria is considered to have uncomplicated malaria (Table 4). Given this information, he should receive atovaquone-proguanil (Table 5).
Severe malaria definition and treatmenta
Treatment of uncomplicated malaria
Most severe malaria cases are caused by P falciparum. Fortunately, our patient appeared to have uncomplicated P falciparum malaria. This could be thanks to acquired immunity from earlier infection, which does not provide sterilizing immunity against parasitemia but may inhibit the development of symptomatic and severe disease. This immunity increases with age, cumulative number of malarial infections, and time spent living in a malaria-endemic area.17 Nevertheless, acquired immunity is usually short-lived without continuous exposure. It is a misconception that prior infection causes lifelong immunity against malaria; in fact, immigrants visiting friends and relatives constitute the most significant group for malaria importation in developed countries.18 Table 6 lists other risk factors for malarial acquisition.
Risk factors for acquiring malaria
If chloroquine phosphate, hydroxychloroquine, quinine, atovaquone-proguanil, or mefloquine is used to treat P vivax or P ovale infection, either primaquine or tafenoquine must be given as presumptive antirelapse therapy (also known as terminal prophylaxis) to prevent late-onset or relapsing disease due to hypnozoites (the liver stage of the parasite) of P vivax or P ovale, which can occur 17 to 255 days after the initial infection.19
The patient was treated with atovaquone-proguanil and recovered.
STAYING HEALTHY ABROAD
4. What can clinicians do to prevent malaria at the present time?
Give chemoprophylaxis that is appropriate to the area the traveler will visit
Instruct patients to take measures to avoid being bitten by mosquitoes
Give the malaria vaccine
Release genetically modified Anopheles to reduce the mosquito population
Malaria prevention
It is essential to give appropriate chemoprophylaxis, taking into account the regions where malarial organisms are resistant to chloroquine, and to instruct patients to take measures to avoid being bitten by mosquitoes.
Risk assessment of travelers to malaria-endemic areas is important (Table 6).20,21 Education of travelers and physicians about chloroquine-resistant areas is essential. Failure to take appropriate precautions may result in death due to severe malaria.22
The US Centers for Disease Control and Prevention (CDC) website provides information on areas with malaria, estimated relative risk of malaria for US travelers, drug resistance, malaria species, and recommended chemopro-phylaxis (Table 7). Some chemoprophylaxis regimens need to be started 1 to 2 weeks before travel to malaria-endemic areas.
Chemoprophylaxis for malaria
Other measures to prevent malaria infection are use of mosquito repellent containing 20% to 35% N,N-diethyl-meta-toluamide (DEET), wearing permethrin-treated clothes, sleeping under insecticide-treated bed nets, and staying in air-conditioned buildings.
Vaccinations
The CDC provides information about vaccinations according to the destination country at wwwnc.cdc.gov/travel. For example, for a traveler going to Ethiopia, vaccinations against cholera, hepatitis A, hepatitis B, meningococcal disease, polio, rabies, typhoid, and yellow fever are recommended.
Certain countries require proof of vaccination against yellow fever to enter, especially if traveling from a country where yellow fever is endemic. Due to limited availability of yellow fever vaccine in the United States, travelers may need to schedule appointments well in advance and visit a nonlocal travel clinic.
Saudi Arabia requires visitors and Hajj and Umrah pilgrims to be vaccinated against meningococcal disease.
Obtaining care abroad
Medical evacuation insurance can be helpful when traveling to a remote destination or to a place where medical care is not up to US standards. Supplemental travel health insurance is recommended as well if the current travel and medical insurance has inadequate coverage.
The US embassy in the destination country (www.usembassy.gov/) can assist in locating medical services and notifying friends and family in the event of an emergency. Other sources such as the International Association for Medical Assistance to Travelers (www.iamat.org/medical-directory; requires free membership login) or International Society of Travel Medicine (www.istm.org/AF_CstmClinicDirectory.asp) can also help you find travel clinics around the globe.
WHAT’S NEW IN MALARIA?
No more quinidine
On March 28, 2019, the CDC issued new guidance for the treatment of severe malaria in the United States. The change in treatment protocol was necessary because quinidine, the only approved intravenous antimalarial drug in the United States, was discontinued by its sole manufacturer, Lilly USA. Previously available lots have now passed their expiration date of March 2019.
Artesunate
Artesunate, the first-line treatment for severe malaria recommended by the World Health Organization, is now the first-line treatment for severe malaria in the United States. However, US clinicians must call the CDC malaria hotline (770-488-7788) to obtain intravenous artesunate.
Malaria vaccine
In 2019, public health programs in Ghana, Kenya, and Malawi began vaccinating young children against P falciparum malaria using the RTS,S/AS01 (RTS,S) vaccine, the first malaria vaccine provided to young children through routine immunization. In an intention-to-treat analysis of a controlled clinical trial, children 6 weeks to 17 months old who received this vaccine had an infection rate of 1.9% compared with 2.8% in a control group that received a nonmalaria comparator vaccine (P < .001), with a number needed to treat of 111 to prevent 1 case of severe malaria.23
Plasmodium and the intestinal microbiome
The intestinal microbiome may influence the development and treatment of malaria. Ippolito et al,24 in a systematic review, discussed how Plasmodium infection may cause intestinal dysbiosis, which correlates with more severe disease outcomes and frequent bacterial coinfection. Moreover, intestinal microbiota may also influence the metabolism of antimalarial agents, susceptibility to Plasmodium in fection, and skin microbiome determinants of mosquito attraction.24
‘Gene-driving’ mosquitoes to be less of a threat
On July 1, 2019, the first release of genetically modified Anopheles mosquitoes in Africa took place in Burkina Faso. This “gene drive” approach, under development at the nonprofit consortium Target Malaria (targetmalaria.org/), is designed to spread mutations through the wild population that knock out key fertility genes or reduce the proportion of female insects that transmit the disease. Researchers released about 10,000 genetically sterilized males to observe their survivability and dispersion in the wild and to introduce the concept of genetically modified mosquitoes to regulators and community members.
Tafenoquine
Tafenoquine was recently approved for treating malaria of all species. It can be used for chemoprophylaxis against all Plasmodium species and, as a single dose, for presumptive antirelapse therapy.25,26 Patients must be tested for glucose-6-phosphate dehydrogenase deficiency before receiving tafenoquine.
CASE CONCLUDED
Our patient recovered from his illness and received education about the importance of malaria chemoprophylaxis when he travels to malaria-endemic areas in the future. The most recent event did not deter him from further travel to buy coffee in South America or Africa; however, he is now an advocate for malaria prevention.
TAKE-HOME POINTS
Fever in a traveler returning from a malaria-endemic area is an emergency.
Clinical features of malaria are nonspecific and include fever, headache, weakness, and profuse night sweats.
P falciparum is chloroquine-sensitive in some areas of Central America and the Caribbean and resistant in all other areas.
A blood smear is the gold standard for diagnosing malaria. However, a rapid diagnostic test can be used if a microbiologist or pathologist is not readily available.
Treatment of malaria depends on the severity and the sensitivity or resistance of the organism in the malaria-endemic area.
- Copyright © 2020 The Cleveland Clinic Foundation. All Rights Reserved.