ABSTRACT
Several key findings in recent years have reshaped our understanding of fibromuscular dysplasia (FMD), an uncommon nonatherosclerotic disease of medium-sized arteries that affects mainly women. While the true prevalence of this disease remains unknown, studies suggest that more people may be affected than previously reported. Better understanding of the clinical manifestations and natural history of FMD and advances in diagnostic imaging have altered the clinical approach to managing patients with this uncommon vascular disease. Although there are a multitude of unanswered questions regarding FMD, this review highlights recent insights and how this information has modified clinical care for those affected.
There is no cure for FMD. Management focuses on thorough evaluation and surveillance, lifestyle modification, and treatment of symptoms. Vascular procedures, such as angioplasty or treatment of aneurysms, are required for some patients.
The overwhelming majority (> 90%) of patients with FMD are women. But men seem to have a more aggressive course, with a rate of aneurysm or dissection two times higher than that in women.
The disease can affect medium-sized vessels throughout the body. In addition to the typical “string-of-beads” appearance or focal lesions, manifestations include arterial tortuosity, aneurysm, and dissection.
Fibromuscular dysplasia (FMD) is an uncommon vascular disease that leads to narrowing (with either a beaded appearance or, less commonly, focal stenosis), dissection, or aneurysm of medium-sized arteries. Awareness of FMD within the medical community has rapidly expanded during the past decade owing to heightened interest among clinicians, multicenter coordinated research initiatives, and patient advocacy efforts.
In addition, a better understanding of the clinical manifestations and natural history of the disease along with advances in diagnostic imaging have altered the clinical approach to management. There are many unanswered questions regarding FMD, but this review highlights recent insights and how this information has modified clinical care for those affected.
DISTINCT FROM ATHEROSCLEROSIS
FMD results from abnormal development of the arterial cell wall, most commonly the vessel media and less commonly the vessel intima (Figure 1).1,2 Distinct from atherosclerotic processes, FMD shares few typical cardiovascular risk factors aside from an association with tobacco smoking.3,4
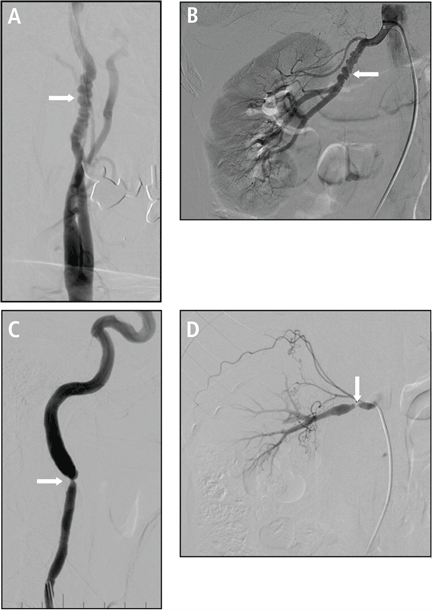
Multifocal fibromuscular dysplasia (FMD) involving the internal carotid artery (A) and a renal artery (B) with a “string-of-beads” appearance. The less common type, focal FMD, involving the internal carotid artery (C) and a renal artery (D).
Reprinted with permission from Wolters Kluwer Health, Inc. (Poloskey SL, et al. Fibromuscular dysplasia. Circulation 2012; 125:e636–e639).
The most common variant of FMD is the multi-focal type, with the affected arteries resembling a string of beads due to alternating regions of stenosis and dilation.1,5 FMD can also cause a singular stenosis (focal type FMD) and has more recently been associated with findings of arterial tortuosity, aneurysm, and dissection.6,7
Though the disease typically affects the renal and extracranial carotid arteries, it has been noted in most medium-sized arteries throughout the body, most commonly the mesenteric, external iliac, and brachial arteries.1 The location of diseased segments determines symptoms, which commonly include hypertension, headache, and pulsatile tinnitus.8 The overwhelming majority of people affected (> 90%) are women.8
The diagnosis of FMD should be suspected in the case of young or middle-aged women presenting with migraine headaches, pulsatile tinnitus, or hypertension and for women with cervical bruits without typical risk factors for atherosclerotic disease. The diagnosis should also be suspected among patients who have suffered an arterial dissection or who are found to have a cerebral, carotid, or renal aneurysm.
THE US REGISTRY FOR FMD
Since it began enrolling patients in 2009, the US Registry for Fibromuscular Dysplasia has grown to include 13 active centers. It collects longitudinal data on the clinical characteristics, presentation, vascular bed involvement, vascular procedures, and clinical outcomes of patients with FMD.8,9 Table 1 highlights key findings and lessons learned from registry publications, many of which have altered previous concepts of this disease.3,7,8,10–12
Key findings of publications from the US Registry for Fibromuscular Dysplasia
EPIDEMIOLOGY AND PATHOPHYSIOLOGY
Prevalence
Although FMD is considered a rare disease (and recognized as such by the National Organization of Rare Diseases), the exact prevalence is unknown. A review of 8 studies conducted from 1963 to 2011 found the prevalence of FMD ranged from 2.0% (3 of 150) to 6.6% (47 of 716) among healthy renal transplant donors for a mean prevalence of 3.3% (268 of 8,029) among all donors.13–21 Findings from the Cardiovascular Outcomes in Renal Atherosclerotic Lesions (CORAL) trial, which studied the effectiveness of medical therapy alone vs medical therapy and stenting for treatment of significant renal artery stenosis and hypertension, found that 5.8% (58 of 997) of participants who underwent angiography had concomitant renal FMD.22 Importantly, patients with FMD were supposed to have been excluded from the trial, suggesting that FMD is often overlooked or underdiagnosed. A review published in 2010 reported the prevalence of cerebrovascular FMD to be 0.3% to 3.2% in patients undergoing cerebral angiography, but it noted significant heterogeneity in patient populations and definitions of FMD across published studies.23
Risk factors for FMD: Female sex and tobacco smoking
The mechanisms underlying the pathogenesis of FMD are still poorly understood, and its development is likely related to a combination of genetic and environmental factors. There seems to be a hormonal component to the pathogenesis of FMD, as most patients with this condition are women: approximately 91.5% of patients enrolled in the US Registry.10 Men with FMD, however, seem to have a more aggressive course with a rate of aneurysm or dissection two times higher than that in women with FMD.7
Studies have reported an increased risk of FMD in patients with a history of tobacco smoking.3,24 A US Registry report notes that FMD patients with a history of smoking had a statistically significant higher rate of aneurysm than those who had never smoked (24.8% vs 18.9%), and there was a trend toward increased prevalence of major vascular events in smokers, including subarachnoid hemorrhage, transient ischemic attack, stroke, mesenteric ischemia, renal infarction, and major coronary event.3 This study also found that patients with FMD who were smokers were more likely to have claudication symptoms (15.1% vs 7.4%) or to have undergone a vascular procedure (45.9% vs 36.7%).3 Further research is needed to fully understand the relationship between smoking and its interaction with other environmental, hormonal, and genetic factors.
FMD and connective tissue features
While studies have suggested a genetic component to the development of FMD, the specific genetic mechanisms are unknown.1 Studies have explored the potential relationship between FMD and genetic connective tissue disorders that can present with vascular manifestations, such as Loeys-Dietz, Marfan, and Ehlers-Danlos syndromes, and isolated case reports have noted concomitant FMD lesions in patients with these classical genetic disorders.25–31 In a series of patients with FMD from Cleveland Clinic who underwent genetic testing for selected connective tissue disorders, including Ehlers-Danlos syndrome and Loeys-Dietz syndrome, the overall yield of these tests was low.31 These studies suggest some overlap of FMD and other vascular connective tissue disorders, as well as the likelihood that the arterial manifestations of FMD may develop through multiple potential genetic pathways.
A series of 47 patients with FMD seen at the National Institutes of Health found a high incidence of connective tissue features on physical examination, with 95.7% of patients exhibiting at least four features of connective tissue disease, including marked hypermobility, scoliosis, craniofacial abnormalities, and pes planus (flat foot deformity).32 A study of a larger cohort of female patients seen at Cleveland Clinic did not find classical connective tissue features (such as pectus deformity, hypermobility, atrophic scaring, and club foot deformity) to a greater extent than what is reported in the general population, but it did find a significant prevalence of severe myopia (near sightedness), high-arched palate, dental crowding, and early-onset arthritis.33 Additional studies are needed to clarify the potential relationship between the spectrum of connective tissue disorders and FMD.
A BROADER SCOPE OF ARTERIAL MANIFESTATIONS
Since FMD was first described in the 1930s,34 most case reports have focused on its renal artery manifestations. In 1964, extrarenal involvement was first reported, which included carotid, iliac, and visceral arteries.35 The medical community has since recognized that the disease can affect medium-sized vessels throughout the body and, more recently, that it is a multifaceted disease with varying arterial manifestations outside of the typical string-of-beads appearance or focal FMD lesions.1 In addition to multifocal or focal narrowings, arterial manifestations of FMD include arterial tortuosity, aneurysm, and dissection.
Arterial tortuosity
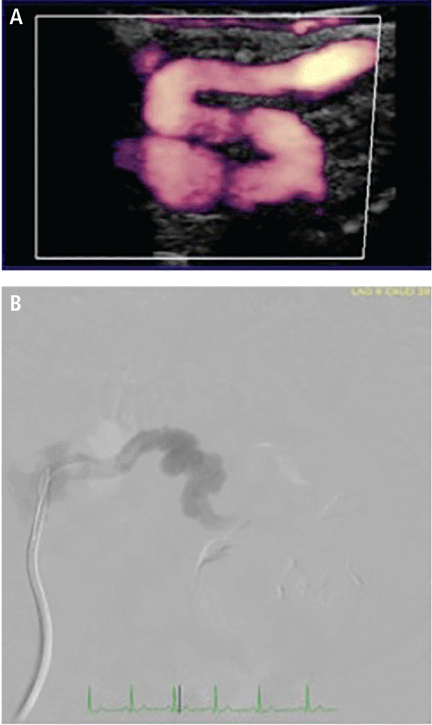
Tortuosity or redundancy of the arteries, particularly the internal carotid arteries, has recently been reported in association with FMD.6 A study based on vascular ultrasonography findings identified this anatomic variant (described as having the appearance of an S-curvature of the internal carotid artery) in 31.9% (37 of 116) of FMD patients.6 This rate of tortuosity is higher than that in the general population, especially when compared with patients of similar age (under age 70). Arterial tortuosity is a common finding in FMD and may be seen in other arterial segments (Figure 2).
(A) Duplex ultrasonography with color power angiography in a patient with fibromuscular dysplasia shows arterial tortuosity (the “S curve”) in the internal carotid artery and areas of beading. This feature can also be seen in renal arteries, as shown on angiography (B).
Aneurysm and dissection
Both arterial aneurysm and arterial dissection are recognized as manifestations of FMD. A US Registry report published in 2016 found a high prevalence of aneurysm and dissection in the FMD population.7 Of the 921 patients included in this analysis, 21.6% had an aneurysm, 25.7% had an arterial dissection, and 41.7% had either aneurysm or dissection. The most common locations for aneurysm were the extracranial carotid, renal, and intracranial arteries, whereas dissection commonly occurred in the extracranial carotid, vertebral, renal, and coronary arteries. The authors noted that these data may be an underestimation, because the entire cohort did not undergo comprehensive screening for asymptomatic aneurysm or dissection. Patients with aneurysm were more likely to have a history of smoking and subarachnoid hemorrhage, while those with dissection were younger and more likely to have headache, neck pain, and end-organ ischemia, including stroke, renal infarction, or myocardial infarction.
FMD of the coronary arteries
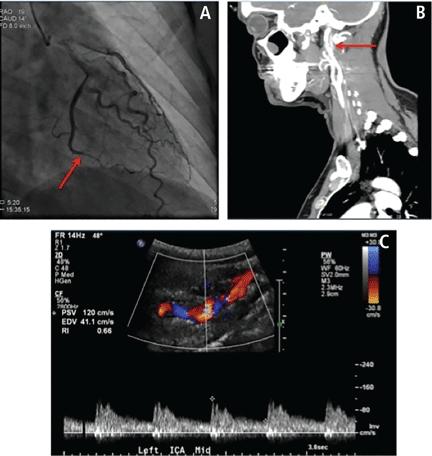
The association between FMD and spontaneous coronary artery dissection (SCAD) has recently been discovered (Figure 3). SCAD typically presents as troponin-positive acute coronary syndrome.36 FMD has been identified as a predisposing condition for SCAD in two case series from Vancouver General Hospital37 and Mayo Clinic.38 The case series from Mayo Clinic found that 45% of SCAD patients had FMD in the extracoronary vessels; the case series from Vancouver General Hospital found that 72% had FMD. A more recent study found that there seems to be other manifestations of FMD in the coronary arteries aside from SCAD.39 In this series, 32 patients with multifocal FMD (in the renal, external iliac, or cerebrovascular arteries) who underwent coronary angiography for suspected symptomatic coronary artery disease (either acute coronary syndrome or stable angina) were found to have coronary artery lesions different from those of atherosclerotic disease. In addition to coronary lesions of dissection (SCAD), the most common findings were marked coronary arterial tortuosity (the “S curve”), followed by areas of atypical-appearing irregular or smooth stenosis. More than half of patients in the series had segments of coronary artery ectasia (enlargement).
Spontaneous coronary artery dissection (SCAD) in a 42-year-old woman with fibromuscular dysplasia (FMD) who presented with chest pain and nausea and non-ST- segment elevation myocardial infarction. She was found with coronary angiography to have SCAD of the left circumflex coronary (A, red arrow). Computed tomographic angiography showed a string-of-beads appearance of the left internal carotid artery (B, red arrow). Duplex ultrasonography showed turbulence and tortuosity in the mid to distal left internal carotid artery, consistent with a diagnosis of multifocal carotid FMD (C).
APPROACH TO MANAGEMENT
There is no cure for FMD, and thus management strategies focus on thorough evaluation and surveillance, lifestyle modification, and treatment of symptoms. Vascular procedures, such as angioplasty or treatment of aneurysms, are required for some patients. Because patients with FMD present with a diverse set of symptoms, consultation with a specialist who has experience with FMD and who works closely with an interdisciplinary team of experts is recommended.1 The interdisciplinary FMD care team may include a vascular medicine physician, cardiologist, nephrologist, neu rologist, neurosurgeon, vascular surgeon, and vascular interventionalist (interventional cardiologist and radiologist).
Imaging and screening the vasculature in FMD patients
Because of the variability in location and manifestations of FMD and the high prevalence of aneurysm and dissection, all patients should undergo comprehensive one-time head-to-pelvis screening during the workup for FMD.1,7 Although the technical standard of diagnostic imaging is catheter angiography, noninvasive imaging—computed tomographic angiography (CTA), magnetic resonance angiography (MRA), duplex ultrasonography—is more commonly used to diagnose and monitor the disease.
A study from our group at Cleveland Clinic assessed the utility of a specialized CTA protocol of the chest, abdomen, and pelvis to image and diagnose manifestations of FMD outside of the cerebrovasculature.40 Incremental findings on imaging included areas of beading or focal narrowing in a new vascular territory and previously undiagnosed arterial aneurysm or dissection. These findings were seen in a variety of vascular beds, including the renal, iliac, and mesenteric arteries, although aortic abnormalities were rare. This study supports the diagnostic value of CTA for FMD to detect asymptomatic aneurysms and areas of arterial dissection, but it also suggests that routine vascular imaging of the thorax may not be necessary.40 In cases of SCAD, on-table renal and iliac angiography (performed after coronary angiography) can assist in diagnosis of FMD as an underlying cause.36 The cerebrovascular arteries (carotid, vertebral, and intracranial vessels) can be imaged later with noninvasive imaging (CTA, MRA).
As a general strategy, once patients with FMD undergo comprehensive imaging, a surveillance program is customized for the patient based on the anatomic location of the disease and the nature of the imaging findings. For example, renal and internal carotid artery FMD may be followed with annual duplex ultrasonography, whereas cerebral and renal or visceral aneurysms require periodic CTA or MRA.
Medical therapies
The medical regimen for patients with FMD varies based on disease location and symptoms, though there are no definitive treatment guidelines because of limited data. A study from the US Registry found that 72.9% of registrants were treated with antiplatelet medications,11 and this is a standard approach in our clinical practice for prevention of thromboembolic events. Antiplatelet drug therapy was more common in elderly patients, patients with a history of coronary artery disease or vascular intervention for FMD, and patients with isolated cerebrovascular FMD.11 Blood pressure management is also important in the medical therapy of patients with FMD who have hypertension. For patients with renal artery involvement, treatment with an angiotensin-converting enzyme inhibitor or angiotensin receptor blocker has been suggested.1
Vascular intervention
The need for vascular intervention (eg, angioplasty or endovascular or surgical aneurysm treatment) is determined primarily by symptoms, with renal artery angioplasty for hypertension the most common FMD-related procedure. It is uncommon for vascular intervention to be performed for cerebrovascular FMD in the absence of recurrent transient ischemic attack or stroke despite antiplatelet therapy, arterial dissection that has failed medical management, or sizable aneurysm that requires treatment to prevent rupture.
When considering intervention for renal artery FMD, it is important to note that the appearance of multifocal FMD (beading) on angiography or noninvasive imaging does not reflect the hemodynamic severity of disease: translesional pressure gradients should be measured across the affected artery to determine if there is actually hemodynamic stenosis caused by an area of beading and to select patients for balloon angioplasty.1 Repeat pressure gradient assessment is done after angioplasty to confirm hemodynamic success.1 Surgical intervention for renal FMD is uncommon. It is generally reserved for complex cases in which endovascular techniques have failed.1
Asymptomatic patients with cerebral, visceral, or arterial aneurysm may require endovascular or surgical treatment. If surgery is indicated, the treatment approach (coiling, stenting, or open surgery) is determined by the size and location of the aneurysm, patient-related factors that may influence the risk of rupture (eg, uncontrolled hypertension, family history of ruptured aneurysm), the anatomic characteristics of the aneurysm, and the feasibility of endovascular vs open surgical repair. A US Registry study of 200 patients with an aneurysm reported that 31.5% underwent intervention to treat the aneurysm.7 Aneurysms requiring intervention were most commonly noted in the extracranial carotid, renal, and intracranial arteries.7
CONCLUSION
Awareness and understanding of FMD have substantially improved in recent years, and this has translated into better care for many patients with FMD. Important advancements have included the recognition of the variability of manifestations of this disease—ranging from an arterial string-of-beads appearance to aneurysm, dissection, and tortuosity— and establishing the need for comprehensive vascular imaging screening in FMD patients. Establishing the association of FMD with SCAD has led to better care for patients with SCAD and presents the opportunity to optimize management of these patients to prevent further vascular events. Research initiatives and heightened awareness have provided valuable insight into this disease, but further work is needed to determine the causal mechanisms of FMD and to continue to develop better management strategies.
Footnotes
Ms. Brinza reported no financial interests or relationships that pose a potential conflict of interest with this article. Dr. Gornik reported membership of the medical advisory board of the Fibromuscular Dysplasia Society of America.
- Copyright © 2016 The Cleveland Clinic Foundation. All Rights Reserved.