ABSTRACT
Point-of-care ultrasonography (POCUS) has emerged as a vital tool in medicine. Initially used for procedural guidance, POCUS is now used for diagnostics and monitoring of the lung, heart, abdomen, and deep vein thrombosis. This wide applicability makes it an essential tool for hospitalists in daily clinical practice. This article provides an overview of the clinical integration of POCUS and basic image interpretation.
Lung POCUS can help in evaluating pneumothorax, alveolar-interstitial syndrome, lung consolidation, and pleural effusions as the cause for respiratory distress.
Focused cardiac ultrasonography can help in evaluating left and right ventricular function, right atrial pressure, pericardial effusion, and tamponade.
Abdominal ultrasonography can aid evaluation of ascites, hemoperitoneum, hydronephrosis, acute pyelonephritis, and gallstones, and can confirm Foley catheter placement.
Point-of-care compression ultrasonography can rapidly detect deep vein thrombosis with high accuracy.
POCUS can guide numerous procedures, including central venous catheter insertion, peripheral intravenous catheter insertion, abdominal paracentesis, and thoracentesis.
Hospitalists are increasingly using point-of-care ultrasonography (POCUS), and have access to ultrasound machines that are more portable, more available, and less expensive. The numerous uses of POCUS for procedural guidance, diagnosis, and monitoring can add considerable value to patient care.
All hospitalists should have an understanding of POCUS nomenclature, applications, and findings. This review highlights various uses of POCUS in hospitalized patients.
DIRECT CLINICIAN INVOLVEMENT
Ultrasonography is low-cost, radiation-free, and noninvasive, allowing it to be repeated multiple times with little risk to patients. What sets it apart from traditional diagnostic ultrasonography is that it is wholly performed by a bedside clinician directly involved in patient care, without requiring a sonographer and radiologist for image acquisition and interpretation (Table 1). A hospitalist can quickly perform a physical examination combined with goal-directed ultrasonography of various organs based on presenting signs and symptoms. Serial scans can be performed to assess progression or response to therapy.
Point-of-care ultrasonography workflow compared with traditional consultative ultrasonography
POCUS enhances patient experience and patient-clinician rapport by increasing interactions between the clinician and patient.1 POCUS has become notably important in the COVID-19 pandemic, allowing protocolized ultrasonographic assessment of multiple organs by a bedside physician, thereby minimizing exposure and the need for formal studies.2
Recognizing the importance of POCUS, numerous medical schools have integrated training in ultrasonography in their curricula. The Society of Hospital Medicine, the American College of Physicians, and the Alliance for Academic Internal Medicine, have also endorsed its use.3–5
Billing for ultrasound-assisted procedures may provide a means to offset the costs of equipment, training, and administration.
IMPROPER USE AND INTERPRETATION CAN CAUSE HARM
POCUS can improve patient care but may also cause harm through improper use and interpretation.6 It needs to be applied in a deliberate and thoughtful manner: multiple views should be obtained for appropriate interpretation, and images must be evaluated in the clinical context. A comprehensive imaging study should be considered if POCUS was of limited utility and the probability of a particular disorder remains high despite negative findings with POCUS.
The accuracy of POCUS depends on the skills and judgment of the operator. Even if basic findings are understood, many nuances and potential pitfalls exist. Clinicians may be falsely reassured by seemingly normal POCUS findings while the patient actually has a serious disease that a radiologic study could detect. Conversely, incidental findings may lead to unnecessary treatments and testing.
But because POCUS may be used improperly does not mean it should not be used. In fact, the major medicolegal issue surrounding POCUS is failure to perform it in a timely fashion.7
LUNG AND PLEURAL ULTRASONOGRAPHY
Lung and pleural ultrasonography can narrow the broad differential diagnosis of respiratory distress (Table 2)8–11 and facilitate prompt management.12 In many hospitals, no radiologist is available to perform lung ultrasonography, making lung and pleural POCUS a critical skill for hospitalists. Training in lung and pleural POCUS is feasible with a simple curriculum consisting of didactics and limited supervised examinations.13,14
Meta-analyses evaluating pleural and lung ultrasonography
Initial lung assessment
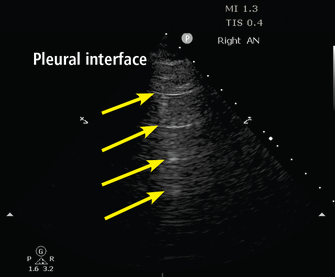
Lung assessment starts with identifying the pleural line, a shimmering hyperechoic structure between the ribs (Figure 1). Respirophasic sliding of the pleura gives a shimmering appearance, referred to as “lung sliding.”
A lines. The A-line pattern occurs in normal lung and in pneumothorax. Ultrasound waves (arrows) reflect off the pleural interface repeatedly, producing repeated horizontal lines throughout the field.
The tissue-air interface in the subpleural region of aerated lung is a strong reflector. Ultrasound is repeatedly reflected between the pleura and the probe, leading to a reverberation artifact appearing as equidistant parallel echoic lines, known as A lines (Figure 1). An A-line pattern indicates normal lung, but it can also be seen with pneumothorax and in conditions with normally aerated pulmonary parenchyma, such as pulmonary embolism, chronic obstructive pulmonary disease, and asthma.15
Evaluation of pneumothorax
In pneumothorax, the air between the parietal and visceral pleurae prevents pleural contact, giving an A-line pattern without lung sliding. Absence of lung sliding has good sensitivity (> 95%) for pneumothorax but poor specificity (60%–99%).8,16 This pattern also occurs with pleural adhesions, apnea, pneumonia, and right mainstem bronchus intubation.16,17 The absence of lung sliding should prompt the search for “lung point,” ie, the transition point at the edge of the pneumothorax where lung sliding is seen in one part and no lung sliding is seen in the rest. Lung point is virtually pathognomonic for pneumothorax (Video 1).17
Video 1. Lung point.
Unlike lung point, absent lung sliding is not specific for pneumothorax and should not by itself prompt tube thoracostomy in the absence of extenuating circumstances (eg, severe cardiorespiratory instability, high clinical suspicion).
Evaluation of alveolar-interstitial syndrome
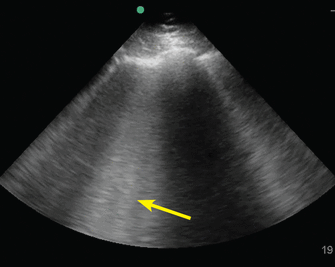
The pathologies of alveolar-interstitial space are characterized by B lines, a sonographic pattern of vertically oriented, laser-like hyperechoic artefacts originating at the pleural interface, extending downwards, and moving synchronously with the pleura (Figure 2). One or 2 B lines are routinely seen; 3 or more in a single field of view is considered abnormal.18–20 Any condition that leads to thickening of subpleural interlobular septa generates B lines, the most common being pulmonary edema (cardiogenic or noncardiogenic in origin).
B lines. The B-line pattern occurs in the setting of interstitial thickening by any cause, including cardiogenic pulmonary edema, noncardiogenic pulmonary edema, interstitial fibrosis, and interstitial pneumonia/pneumonitis. It is analogous to ground-glass opacity on computed tomography. It is demonstrated by vertical lines resembling the tail of a comet and extending to the bottom of the screen. In this image, confluent B lines (arrow) indicate significant interstitial involvement.
The sensitivity of B lines in identifying pulmonary edema is at least 90%,9,21,22 making POCUS an excellent tool to differentiate cardiogenic pulmonary edema from exacerbation of chronic obstructive pulmonary disease. In addition, B lines may help guide diuresis and assess fluid tolerance.
B lines also occur in pneumonia, pulmonary fibrosis, acute respiratory distress syndrome, and pneumonitis of any etiology. Careful evaluation of the pattern of B-line distribution and the pleural line, along with clinical correlation, can help distinguish these different causes (Table 3).23–25
Characteristics of B lines based on etiologya
The presence of B lines effectively rules out pneumothorax, as they are produced from subpleural lung units.17
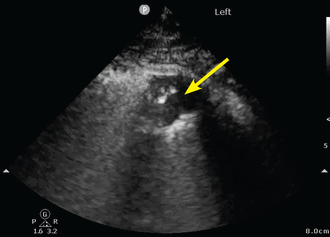
Evaluation of consolidation
Ultrasound waves can traverse subpleural lung consolidation, resulting in the absence of A lines and a true 2-dimensional image of the consolidated lung (Figure 3). Almost all acute alveolar consolidations (98.5%) are found adjoining the visceral pleura, providing the necessary window for detection.26
Small peripheral (subpleural) consolidation. This is demonstrated by a small area of lung parenchyma visualized directly beneath the pleura (arrow). This pattern is common in bacterial or viral pneumonia, including COVID-19 pneumonia.
The finding of subpleural consolidation or focal B lines, or both, is suggestive of pneumonia. The sensitivity and specificity of lung ultrasonography for diagnosing pneumonia is just 85% or more.10 Nonetheless, supportive clinical and laboratory data with the characteristic ultrasound patterns can substantiate a diagnosis of pneumonia.
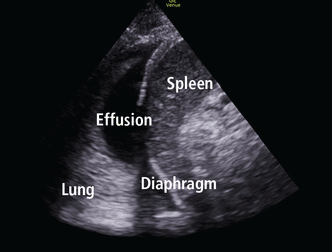
Evaluation of pleural effusion
Portable chest radiography has a sensitivity of 60% for detecting pleural effusion27; in contrast, lung ultrasonography is 94% sensitive and 98% specific.11 Lung ultrasonography can also better characterize basal opacities by distinguishing consolidation from pleural effusion (Figure 4). It can also detail the features of pleural effusion, with simple effusion appearing anechoic, and complex effusions characterized by septations, loculations, and debris. The size of a pleural effusion can also be quantified using lung ultrasonography.28
Pleural effusion and consolidation.
FOCUSED CARDIAC ULTRASONOGRAPHY
Focused cardiac ultrasonography (this term is preferred to “echocardiography” to highlight its focused nature) provides critical insight into hemodynamic status. It can be performed with excellent diagnostic accuracy for important cardiac abnormalities (Table 4).29
Focused cardiac ultrasonography: Basic views and key findings
Focused questions, including global assessment of left ventricular function, presence or absence of a pericardial effusion, assessment of right ventricular size and function, and estimation of right atrial pressure, can help narrow a differential diagnosis and guide management in patients with cardiorespiratory distress.
Evaluating left ventricular function
Evaluation of left ventricular systolic function is one of the primary objectives of focused cardiac ultrasonography. As a general rule, multiple views should be obtained for appropriate interpretation. Although objective methods of left ventricular systolic evaluation are available and recommended, qualitative “eyeball estimation” is appropriate and feasible, with studies demonstrating high accuracy of visual estimation compared with recommended objective measures.30,31 Left ventricular systolic function can be qualitatively graded as severely reduced, moderately reduced, mildly reduced, normal, or hyperdynamic. Cardiology-performed echocardiography can be requested for further quantitative evaluation (Video 2, Video 3).
Video 2. Normal parasternal long axis view.
Video 3. Reduced ejection fraction.
Evaluating right ventricular function
Better understanding of the importance of right ventricular function has led to including its evaluation in various protocols assessing shock and respiratory failure.32 Although objectively estimating right ventricular size and function is challenging, qualitative assessment can be made at the bedside by directly comparing the left and right ventricle.
Size. The right ventricle is normally less than two-thirds the size of the left. A right ventricle-to-left ventricle ratio of 1 or higher is associated with poor outcomes in pulmonary hypertension, pulmonary embolism, and other critical conditions.
Septal kinetics. Assessing septal kinetics can also provide vital insights and help identify the cause of right ventricular dysfunction: septal deviation occurs toward the left ventricle in diastole with right ventricular volume overload, and during systole with right ventricular pressure overload.
Chronicity. It is important to distinguish acute from chronic right ventricular dysfunction, as their causes differ. Distinguishing them is challenging with focused cardiac ultrasonography, yet certain subtle findings can point to the cause.
Chronic dysfunction is seen in long-standing cases of pulmonary hypertension. It is associated with right ventricular hypertrophy with right ventricular free-wall thickness of more than 5 mm (Video 4).
Video 4. Dilated right ventricle.
Acute dysfunction raises concern for massive pulmonary embolism, acute respiratory distress syndrome, and acute right ventricular infarction. In acute right ventricular dysfunction, particularly pulmonary embolism, the McConnell sign (ie, right ventricular free-wall akinesis with sparing of the apex) is just 70% sensitive and 33% specific for diagnosing acute pulmonary embolism (positive likelihood ratio [PLR] 1.04, negative likelihood ratio [NLR] 0.91).33 Hence, pulmonary embolism cannot be definitively diagnosed with focused cardiac ultrasonography, with the notable exception of detecting a visible thrombus in the right heart (ie, a clot in transit).
Evaluating valvular abnormalities
Limited evaluation of the mitral, tricuspid, and aortic valves can be performed using standard views. With some experience, gross abnormalities that may significantly alter management (eg, flail leaflet, prolapse, large vegetation, chordae rupture) can be detected on visual examination and color Doppler. Dynamic left ventricular outflow tract obstruction due to systolic anterior motion of the mitral valve can be detected visually and by using motion mode (M mode). Although systolic anterior motion is classically seen with hypertrophic cardiomyopathy, it may also occur in other situations that lead to worsening hemodynamics (eg, sepsis, acute hemorrhage, dehydration). Systolic anterior motion may be associated with severe mitral regurgitation, which resolves with resolution of systolic anterior motion.
However, bedside echocardiography is limited for assessing valvular pathologies. A detailed assessment of valvular lesions (especially stenotic lesions) involves use of spectral Doppler in multiple views, which is not part of basic cardiac ultrasonography. Hence, a comprehensive echocardiographic examination should be considered for evaluating valvular abnormalities and pathology.34
Estimating right atrial pressure
In spontaneously breathing patients, right atrial pressure can be estimated by measuring inferior vena cava size and collapsibility with deep inspiration or “sniff” (Table 5).35 The influence of respiratory effort, intra-abdominal pressure, and positive-pressure ventilation may limit the accuracy of the measurement and should be considered. Additionally, the long-axis view of the inferior vena cava is prone to error due to off-plane assessment and respirophasic movement. This can be overcome by acquiring a short-axis (transverse) view.36
Estimates of central venous pressure based on inferior vena cava size and collapsibility
Evaluating pericardial effusion and tamponade
Focused cardiac ultrasonography has excellent sensitivity (96%) and specificity (98%) for detecting pericardial effusion (PLR 48, NLR 0.04)37 and can trigger further consultation for evaluation of tamponade, if clinically suspected (Video 5, Table 4). Hemodynamic instability from cardiac tamponade results from increased pericardial pressure, impairing venous return. The rate of fluid accumulation plays a more prominent role than size in tamponade physiology. Thus, a large volume of pericardial effusion can accumulate over time without impairing hemodynamics, while a smaller pericardial effusion or hemorrhage in the setting of trauma or postprocedure can lead to the need to diligently inspect echocardiographic signs of tamponade.
Video 5. Pericardial effusion and tamponade.
A plethoric inferior vena cava from impaired filling is highly sensitive (92%) but not specific for cardiac tamponade. Right atrial collapse for more than one-third of the cardiac cycle is highly sensitive and specific for diagnosing tamponade, followed by right ventricular collapse during diastole.38 Absence of chamber collapse has a negative predictive value of 90%. Assessing for tamponade can be difficult, and M mode may help identify chamber collapse. Concerning or indeterminate findings for tamponade should prompt urgent expert consultation or a confirmatory echocardiogram, or both.
POCUS has been shown to reduce time to pericardiocentesis and is recommended to guide drainage of effusion.39
Common pitfalls of focused cardiac ultrasonography
Focused cardiac ultrasonography is prone to the following common issues:
Not obtaining a complete echocardiogram when needed. A focused study serves a different purpose from a complete study and should not replace one. Hence, a “normal” focused cardiac ultrasonographic evaluation does not obviate the need to order a complete transthoracic echocardiogram that is clinically indicated.
Over-relying on POCUS to manage volume. POCUS findings are useful as part of volume status assessment, but a single POCUS finding in isolation should not be used to determine volume management (eg, giving fluids for an apparently “collapsed” inferior vena cava). Findings are prone to variability and must be integrated into overall assessment, not used in isolation.
Delaying POCUS during shock. Focused cardiac ultrasonography should be performed promptly in a patient with shock. Not doing so may lead to an important missed diagnosis, such as pericardial tamponade, ventricular dysfunction, or valvular abnormality.
ABDOMINAL ULTRASONOGRAPHY
Evaluation of ascites and hemoperitoneum
Evaluating thoracoabdominal trauma is often a diagnostic challenge, prompting clinicians to depend on ancillary tests to detect potentially life-threatening internal injuries. Ultrasonographic evaluation of free fluid in the abdomen has been extensively studied in trauma literature for detecting hemoperitoneum. Today, ultrasonography has virtually replaced diagnostic peritoneal lavage as a primary, bedside imaging method for trauma patients.40 Numerous studies have found that examinations performed and interpreted by treating physicians are reliably accurate compared with those read by radiologists.41
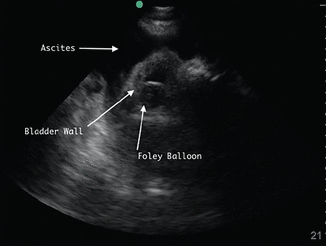
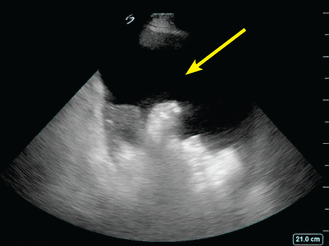
POCUS can also help hospitalists detect ascites. It is more sensitive and specific than physical examination and can guide the decision to perform paracentesis (Figure 5, Figure 6).42
Right lower quadrant with large ascites fluid pocket; Foley catheter in bladder.
Ascites pocket.
Evaluation of kidney and bladder
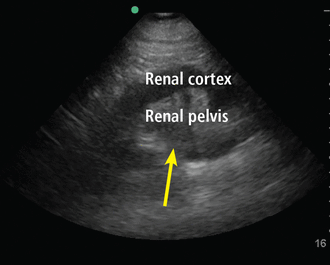
Hydronephrosis, a commonly encountered and often reversible cause of acute kidney injury, can be detected with high sensitivity and specificity by a bedside clinician using POCUS (Figure 7).43 Hydronephrosis results from urinary flow obstruction, which can be internal (eg, from ureteral calculus or a mass) or external (eg, from ureteral compression from structures such as an enlarged abdominal aortic aneurysm, an advanced pregnancy, or a pelvic mass). Evaluation for hydronephrosis can be useful in cases in which urinary obstruction is considered. This may be particularly important in patients with acute pyelonephritis. However, mimics of hydronephrosis include prominent renal pyramids, prominent renal vasculature, and parapelvic cysts.
Hydronephrosis. Hypoechoic (dark) fluid (arrow) is shown extending into the renal pelvis.
Distal obstruction (eg, prostatic hypertrophy) usually results in bilateral hydronephrosis, so it is important to scan both kidneys.
A study found more than 90% sensitivity and specificity for detecting hydronephrosis by POCUS performed by internal medicine residents given 5 hours of training compared with comprehensive radiologic ultrasonography.44
POCUS is also helpful in acute pyelonephritis to evaluate for obstruction. Detecting large obstructive calculi would prompt urgent urologic consultation.
A distended bladder, being a large fluid-filled structure, is easily visualized by ultrasonography and can be distinguished from ascitic fluid. POCUS can be used to estimate bladder volume and confirm proper placement of a urinary catheter by visualizing a Foley balloon inside the bladder (Figure 5). This application may be particularly useful in a patient with obesity or ascites, which can make physical examination or bladder scanner determinations inaccurate. In patients without a urinary catheter, bladder volume estimation should be performed post-void.
Ultrasound evaluation of the biliary system
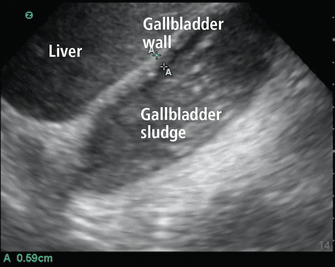
Gallstones appear by ultrasonography as round hyperechoic structures in the gallbladder or bile ducts, with posterior acoustic shadowing. POCUS has demonstrated excellent sensitivity (89.8%) and specificity (88.0%) for detecting cholelithiasis (PLR 7.48, NLR 0.12).45 Findings suggestive of acute cholecystitis include gallstones, pericholecystic fluid, gallbladder wall thickening, and sonographic Murphy sign (ie, abdominal pain elicited by probe pressure), all of which can be assessed at the bedside with good specificity (Figure 8).46
Gallbladder containing sludge, with a thickened anterior wall, in a patient with acute cholecystitis.
The common bile duct can also be measured by POCUS, although it is technically challenging, especially for a novice user.47 Requesting a formal ultrasonographic study is prudent to obtain this information.
EVALUATION OF LOWER-EXTREMITY DEEP VEIN THROMBOSIS
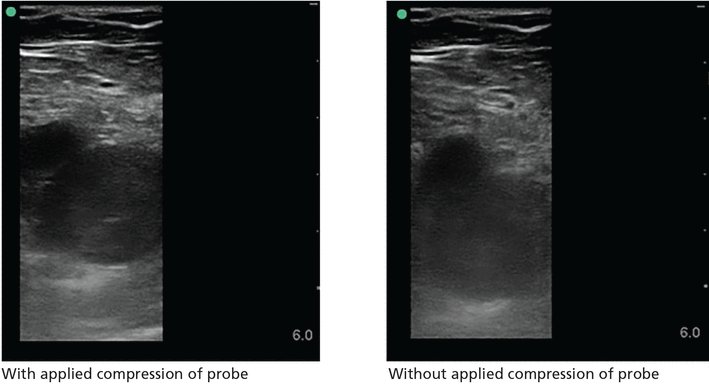
Although complete duplex ultrasonography is the standard radiological study traditionally performed to evaluate for deep vein thrombosis (DVT), point-of-care compression ultrasonography can be performed rapidly with high diagnostic accuracy after limited training (Figure 9).48 A multicenter study of hospitalist-performed compression ultrasonography found a sensitivity of 100% and specificity of 96% for identifying lower extremity DVT, reducing the time to diagnosis by nearly 5 hours compared with corresponding vascular studies interpreted by radiologists.49 Meta-analyses have also reported sensitivity and specificity higher than 90% (Table 6).50–52 However, inadequate compression, lymph nodes, Baker cysts, and superficial venous thrombosis may be mistaken for a DVT.
Right common femoral vein deep vein thrombosis. The left image shows lack of compression of the vein with applied compression of the probe. The right image shows vein without compression.
Meta-analyses evaluating point-of-care ultrasonography for diagnosing deep vein thrombosis
A focused DVT study is performed using a high-frequency (5–12 MHz) linear probe with compression of the vein at multiple sites, traditionally using a 2-point (common femoral vein and popliteal vein) or 3-point (same, plus superficial femoral vein) method. The 3-point examination demonstrated higher sensitivity (91% vs 83%) and similar specificity (96%) to the 2-point examination, but it still can miss 5% of isolated femoral vein DVTs.53 An extended compression examination employing compressing the femoral vein every 2 to 3 cm until it dives into the adductor canal and popliteal vein along its course is more comprehensive and is currently the recommended method.3
Duplication of the venous system in the lower extremity is common, and the presence of a DVT in duplicated systems could easily be missed.54 A positive POCUS examination may prompt early initiation of anticoagulation and ordering of confirmatory imaging; a negative POCUS test in a patient with high pretest probability needs a comprehensive vascular study. The negative predictive value for a POCUS DVT study is not sufficient to effectively rule out DVT in such patients.
EVALUATING SKIN AND SOFT-TISSUE INFECTIONS
The major role of POCUS in evaluating skin and soft-tissue infection is to detect abscess formation in the soft tissue. It has been found to change management in more than half of patients presenting with a skin or soft-tissue infection.55
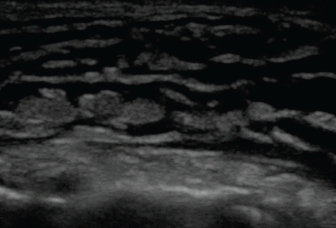
The cobblestone appearance of cellulitis (Figure 10) is nonspecific and can be seen in any cause of subcutaneous edema, while ultrasonography is 98% sensitive and 88% specific for abscess detection (PLR 8.17, NLR 0.02).56 The appearance of abscesses ranges from anechoic to hyperechoic and may demonstrate posterior acoustic enhancement.
On ultrasonography, subcutaneous fluid is demonstrated as hypoechoic or anechoic (dark) layering within islands of subcutaneous tissue (gray). This occurs in any process leading to fluid within the subcutaneous tissue, including cellulitis and hydrostatic edema.
Color Doppler is highly informative, as abscess cavities do not have internal Doppler flow. The presence of flow within the cavity may suggest a vascular structure such as a pseudoaneurysm. Air within the cavity, suggestive of high-grade infection, can be easily detected with ultrasonography.
Ultrasonography may also help diagnose necrotizing fasciitis by detecting fascial and subcutaneous thickening, abnormal fluid accumulation in the deep fascia layer, and subcutaneous air. However, ultrasonography should not be used to rule out the diagnosis of necrotizing fasciitis.
ULTRASONOGRAPHY FOR PROCEDURAL GUIDANCE
Numerous procedures common to hospital medicine practice may be performed more safely and effectively with ultrasonography. The Society of Hospital Medicine has published recommendations for the use of ultrasonography in common hospital medicine procedures, including abdominal paracentesis,42 thoracentesis,57 lumbar puncture,58 and venous access,59 as well as for procedural credentialing.60
Procedures may be “ultrasound-assisted” or “static” (ie, ultrasonography is used for site selection, then the procedure is performed without ultrasonography) vs “ultrasound-guided” or “dynamic” (ie, the procedure is performed with live ultrasonographic guidance, with the ultrasound probe in one hand and a needle in the other).
Central venous catheter insertion
For central venous catheter insertion, ultrasonography reduces time to completion and decrease failed attempts, with fewer complications like pneumothorax and arterial punctures. It also aids in preprocedural detection of stenosis and thrombosis of the target vein, and it is currently the standard of care for upper-extremity central venous catheter insertion.61 Nonetheless, this procedure remains highly user-dependent, and adequate training is critical.62
Peripheral intravenous catheter insertion
Ultrasonography is increasingly used to guide peripheral intravenous catheter insertion. In addition to increasing patient satisfaction, it has demonstrated a higher success rate, particularly in patients with difficult access, reducing the need for a central venous catheter. Ultrasonography can also be used to confirm the correct placement by visualizing the catheter in the vein or detecting bubbles with saline flush.63
Abdominal paracentesis
Ultrasonographic guidance of paracentesis has been found to have a 95% success rate compared with 61% using the traditional landmark-based method.64 Unsurprisingly, paracentesis was successfully completed with ultrasonography in 87% of the patients for whom the landmark method failed. In a large observational database study of 70,000 patients undergoing paracentesis, ultrasonographic guidance significantly reduced bleeding complications.65,66
In addition, a linear probe can help identify underlying vasculature, including the inferior epigastric artery, further minimizing major bleeding risk.
Thoracentesis
Ultrasonography has also demonstrated a higher rate of success and fewer complications for thoracentesis. In a meta-analysis of 24 studies with 6,605 thoracentesis procedures, ultrasonography significantly reduced pneumothorax compared with the landmark technique, even with inexperienced operators.67 The procedure can be performed using static or dynamic ultrasonographic guidance. If static technique is used, the patient position needs to be maintained after marking the spot.
Evaluation of normal lung sliding preprocedure and postprocedure obviates the need for chest radiographs to rule out pneumothorax.57
Common pitfalls
Ultrasound gel can prevent effective preprocedural aseptic skin preparation and postprocedural dressing adherence. Gel should dry before cleaning the skin or applying a dressing.
In addition, use of ultrasonography may sometimes lead to failure to look at anatomical landmarks, leading to performing a procedure at a nonideal site. Users should be mindful of anatomic landmarks in addition to sonographic features.
CONCLUSION
The role of bedside ultrasonography has undergone a paradigm shift, with a variety of applications being explored. This shift has been driven by the realization that performance of POCUS is a readily achievable skill and is rewarding in daily practice. It is no surprise that many predict that it will be the standard of care in the near future. Hospitalists are at the forefront of patient care and should be cognizant of the many benefits of POCUS. We hope that wider utilization of ultrasonography at the bedside can improve medical decision-making, translating to better patient care.
DISCLOSURES
The authors report no relevant financial relationships which, in the context of their contributions, could be perceived as a potential conflict of interest.
- Copyright © 2021 The Cleveland Clinic Foundation. All Rights Reserved.