ABSTRACT
Esophageal cancer is the sixth most common cause of cancer-related death worldwide. Esophageal adenocarcinoma is the most common subtype of esophageal cancer in the United States, and its incidence has risen dramatically in the last few decades. Modern endoscopic and surgical techniques have significantly improved morbidity and mortality rates of patients undergoing treatment for esophageal cancer. However, most cases are diagnosed at a late stage when the prognosis is poor, emphasizing the need for an effective screening strategy. This clinical overview focuses on screening, multidisciplinary evaluation, and treatment of early esophageal adenocarcinoma.
The 2 major subtypes of esophageal cancer are squamous cell carcinoma and adenocarcinoma, and they have different clinical presentations and natural history. The incidence of adenocarcinoma of the esophagus has increased dramatically over the past few decades in the Western world.
There are currently no standard or routine screening tests for esophageal cancer. However, many tests are under investigation for screening patients at high risk.
Management of early esophageal adenocarcinoma is based on patient and tumor characteristics and available institutional expertise.
Esophageal cancer is the sixth most common cause of cancer-associated death worldwide, accounting for an estimated 1 in every 20 cancer deaths.1 More than 500,000 new cases are reported every year.1
Worldwide, squamous cell carcinoma is the most common type of esophageal cancer, followed by adenocarcinoma, while small-cell carcinoma, melanoma, sarcoma, and lymphoma are rare. However, in Western countries, esophageal adenocarcinoma is much more common than esophageal squamous cell carcinoma (Table 1),2 and its incidence is rapidly growing in developed countries owing in part to the rising prevalence of obesity and gastroesophageal reflux disease.
Esophageal adenocarcinoma vs squamous cell carcinoma
Esophageal adenocarcinoma has a favorable prognosis if diagnosed early, when it is isolated to the mucosal and submucosal layers of the esophagus. Unfortunately, most cases are diagnosed at a late stage, when the prognosis is dismal. The 5-year overall survival rate of patients with esophageal adenocarcinoma is less than 20%, comparable to that of patients who have liver, lung, or pancreas cancer.3 Thus, there is a dire need for effective screening strategies to diagnose it earlier.
Treatment has primarily focused on resection, either surgical or, more recently, endoscopic. Radiation therapy and chemotherapy have historically been considered in patients in whom resection is less feasible because the cancer has already spread. For esophageal cancer in general, a multidisciplinary approach may help identify the best therapeutic strategy based on patient and tumor characteristics and local expertise.
This review provides strategies relevant to the subset of esophageal adenocarcinoma that is detected early, and highlights the need for a multidisciplinary approach.
RISK FACTORS
Obesity
A meta-analysis of over 16,000 cases confirmed a strong association between body mass index, obesity, and esophageal adenocarcinoma.4
Multiple risk factors
In another study, the prevalence of Barrett esophagus (the precursor lesion of esophageal adenocarcinoma) was found to have a positive linear relationship with the number of risk factors, which included gastroesophageal reflux disease, male sex, age over 50, family history of Barrett esophagus or esophageal adenocarcinoma, and obesity (defined as body mass index > 35 kg/m2).5
Other, unreliable factors
Symptoms. Most patients with early-stage esophageal adenocarcinoma are over age 65 and have no symptoms. The esophagus, being a distensible tube, can accommodate smaller tumors that remain asymptomatic until the lesion grows to a significant size.
Since gastroesophageal reflux disease involves mostly the distal esophagus and gastroesophageal junction, 94% of cancers associated with Barrett esophagus are found below the tracheal bifurcation. Significant dysphagia in early lesions should raise suspicion of more advanced disease or, rarely, a concurrent nonmalignant cause such as peptic stricture, inflammation, or concurrent submucosal tumor.
Eosinophilic esophagitis causes chronic inflammation of the esophagus, raising concerns that it may increase malignant transformation. However, a recent large database study could find no relationship between eosinophilic esophagitis and esophageal cancer.6
Alcohol consumption does not appear to increase the risk of esophageal adenocarcinoma, and some studies suggest wine may actually be protective.7
WHO SHOULD BE SCREENED?
Barrett esophagus, the major precursor of esophageal adenocarcinoma, is believed to progress through pathologic stages, from metaplasia to low-grade dysplasia, high-grade dysplasia, and esophageal adenocarcinoma. The rise in esophageal adenocarcinoma and its poor prognosis in its advanced stages have raised interest in screening for Barrett esophagus and following it closely when discovered.8
In a prospective study, when patients with Barrett esophagus underwent endoscopic surveillance, the cases of esophageal cancer that arose were diagnosed at an earlier stage than in the general population.9 However, studies have failed to identify an accurate, cost-effective, widely applicable tool that can lower the mortality rate.
Current guidelines, which are based on low-quality evidence and expert opinion, restrict screening to a very specific patient population: ie, those with long-standing gastroesophageal reflux disease (> 5 years) and those with frequent reflux symptoms (weekly or more) with 2 or more risk factors for Barrett esophagus or esophageal adenocarcinoma.10 These risk factors include male sex, age over 50, central obesity (a waist circumference > 102 cm or a waist-hip ratio > 0.9), current or past history of smoking, White race, first-degree family history of Barrett esophagus or esophageal adenocarcinoma, or hiatal hernia. Patients diagnosed with Barrett esophagus without dysplasia should undergo endoscopy every 3 to 5 years.
In a large nationwide study, the annual risk of esophageal adenocarcinoma after a diagnosis of Barrett esophagus was 0.12%, much lower than the assumed risk of 0.5%, which is the basis for current guidelines.11 However, nearly 90% of cases of esophageal adenocarcinoma are diagnosed in patients not known to have Barrett esophagus.12 This shows that the current screening guidelines continue to miss a large number of patients at risk.
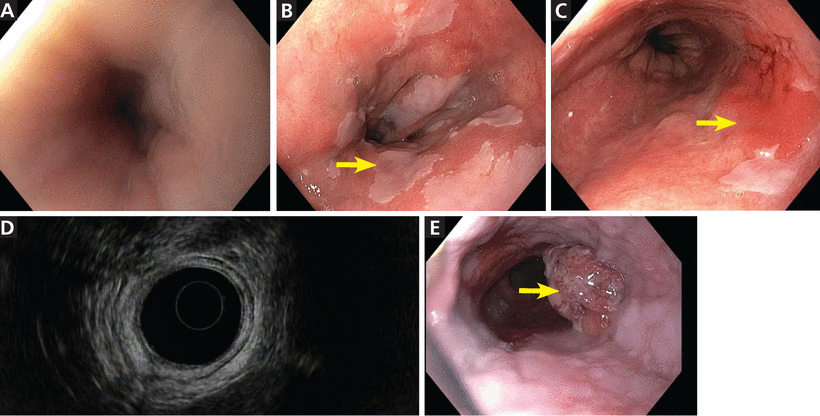
Upper endoscopy (Figure 1) is the gold standard for screening, but it necessitates sedation and is relatively expensive and inconvenient for a screening procedure. An ideal screening tool needs to be relatively inexpensive, well-tolerated, and applicable to general practice.
Endoscopic views of the esophagus. (A) Normal esophagus. (B) Barrett esophagus with islands of normal squamous mucosa (arrow). (C) Barrett esophagus with a discrete erythematous mass 4 × 2 cm (arrow) in the involved segment. (D) Barrett esophagus, endoscopic ultrasonographic view. (E) Esophageal adenocarcinoma (arrow).
Detection rates of Barrett esophagus have been improved with advances in endoscopy such as high-definition imaging, chromo-endoscopy (which uses special staining to enhance mucosal visualization), and narrow-band imaging (which enhances the mucosal resolution by selecting specific wavelengths of light).
Swallow studies such as barium swallow do not allow for histologic assessment for metaplasia or dysplasia. Therefore, they must not be used for screening or surveillance of Barrett esophagus.
Newer screening methods for Barrett esophagus
Screening methods for Barrett esophagus that do not require endoscopy with sedation are under investigation.
Cytosponge (Medtronic) is an ingestible capsule containing a sponge attached to a string. The capsule dissolves on reaching the stomach and releases the sponge, which can be withdrawn from the esophagus out of the mouth by pulling the string. The sponge collects epithelial cells on its way out of the esophagus and is then tested for biomarkers of Barrett esophagus such as trefoil factor 3. Cytosponge is inexpensive and safe, and a prospective study found it to have a sensitivity of 73% and a specificity of 94% for detecting lesions measuring at least 1 cm.13 A systematic review had similar findings.14
A swallowable balloon device can similarly sample the distal esophagus and detect DNA methylation markers. Its reported sensitivity in detecting Barrett esophagus metaplasia was 90.3% and its specificity 91.7%.15
Transnasal endoscopy, another office-based technique, uses a reusable endoscope with a disposable outer sterile sheath. It seems to be better tolerated than standard endoscopy while showing similar findings.16
Breath testing using an “electronic nose” to detect volatile organic compounds in exhaled air has shown promising results, with a sensitivity of 91% and specificity of 74%.17
These novel screening tools may prove to be efficient and cost-effective in primary care. However, more research is needed before they can be widely adopted. Clinical trials are under way to assess patient acceptance and preference for these different tools.
Possible preventive measures
Although epidemiologic studies suggested aspirin and nonsteroidal anti-inflammatory drugs might prevent Barrett esophagus and esophageal adenocarcinoma, clinical trials of these drugs to prevent esophageal adenocarcinoma have been unsuccessful.18
Retrospective data from multiple centers show that diets rich in antioxidants, fruits, vegetables, omega-3 fatty acids, polyunsaturated fat, and fiber are associated with lower risk of Barrett esophagus.19,20
BIOPSY IS THE GOLD STANDARD FOR DIAGNOSIS
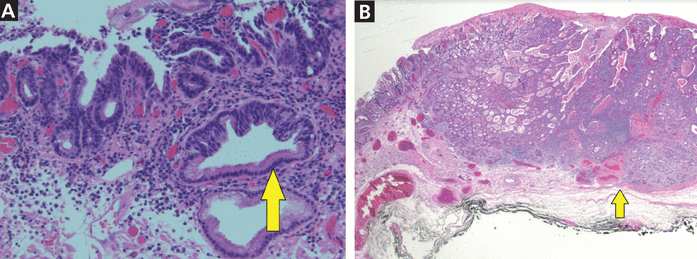
On endoscopy, early lesions of esophageal adenocarcinoma can be flat, polypoid, or slightly depressed. Advanced tumors present as masses that may obstruct the esophageal lumen. The gold standard for diagnosing esophageal adenocarcinoma is tissue sampling by endoscopic biopsy (Figure 2). A prospective trial revealed a diagnostic accuracy of 93% with a single biopsy, and additional biopsy specimens increased the yield to over 98%.21
(A) High-grade dysplasia (arrow) from the periphery of a Barrett esophagus lesion (hematoxylin and eosin, magnification × 4). (B) Complex atypical glandular proliferation diagnostic of adenocarcinoma and involving the submucosa (arrow highlights submucosa) (hematoxylin and eosin, magnification × 20).
CANCER STAGING IS PARAMOUNT
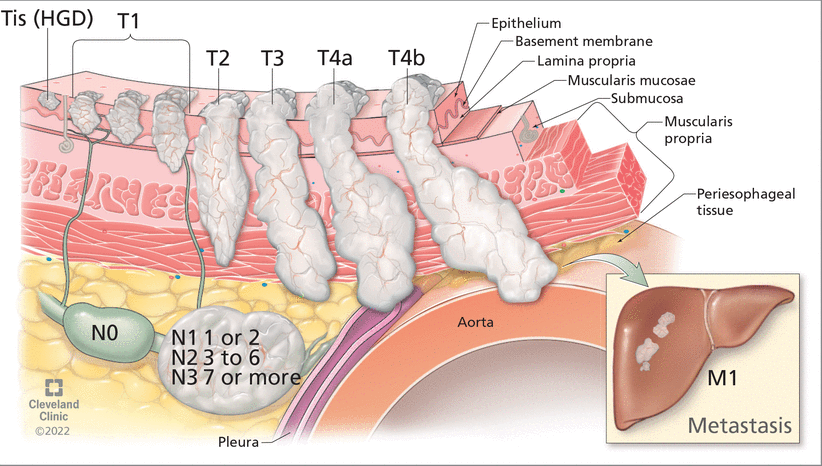
Once esophageal adenocarcinoma is diagnosed, its stage needs to be assessed to determine prognosis and treatment. This involves the TNM system (Figure 3), as follows:
Tumor depth (categorized on a scale of Tis through T4b)
Nodes, ie, number of lymph nodes affected (categorized on a scale of N0 through N3)
Metastasis in distant organs (M0 for no distant metastasis, or M1 for distant metastasis).
The tumor, node, metastasis (TNM) staging system for esophageal cancer helps determine prognosis and treatment based on tumor depth, number of affected lymph nodes, and metastasis to distant organs.
HGD = high-grade dysplasia
Positron emission tomography with computed tomography. The role of 18-fluorodeoxyglucose (FDG) positron emission tomography with computed tomography (PET/ CT) and endoscopic ultrasonography in early esophageal adenocarcinoma staging is controversial. However, the National Comprehensive Cancer Network guidelines22 recommend staging by PET/CT and endoscopic ultrasonography in cases of advanced cancer (≥ T1b) to evaluate for nodal spread.
PET/CT is less beneficial in early esophageal adenocarcinoma than in advanced disease. Some studies found that it could not reliably detect early esophageal adenocarcinoma stages such as T1a and T1b tumors.23,24 A study of 79 patients with clinically staged T1a and T1b esophageal adenocarcinoma who underwent preoperative PET/CT showed all FDG-avid nodes seen were false positives23; another study had similar findings.24 This suggests that PET/CT could lead to more unnecessary biopsies. However, if a tumor is found to be more advanced on pathologic study after endoscopic submucosal dissection, performing PET/CT after resection has limited utility, as inflammation of the resection bed is often FDG-avid on PET.
For this reason, we consider PET/CT before resecting bulky or borderline tumors larger than 15 mm or lesions with suspected superficial submucosal invasion (SM1) greater than 500 μm.
Endoscopic ultrasonography can assess for the depth of tumor invasion and locoregional lymph node spread. However, it has a high false-positive rate of up to 10%.25 Consequently, the American Society for Gastrointestinal Endoscopy guidelines strongly recommend against its routine use in early esophageal adenocarcinoma to stage mucosal (T1a) and submucosal (T1b) disease.10
These days, more advanced tumors are being referred for endoscopic resection. Thus, accurate staging and ruling out advanced disease before proceeding with endoscopic treatment is paramount. Further research is required to understand the role of PET/CT and endoscopic ultrasonography in large T1a (> 15 mm) and early T1b disease that is increasingly being elected for endoscopic resection.
TREATMENT OPTIONS
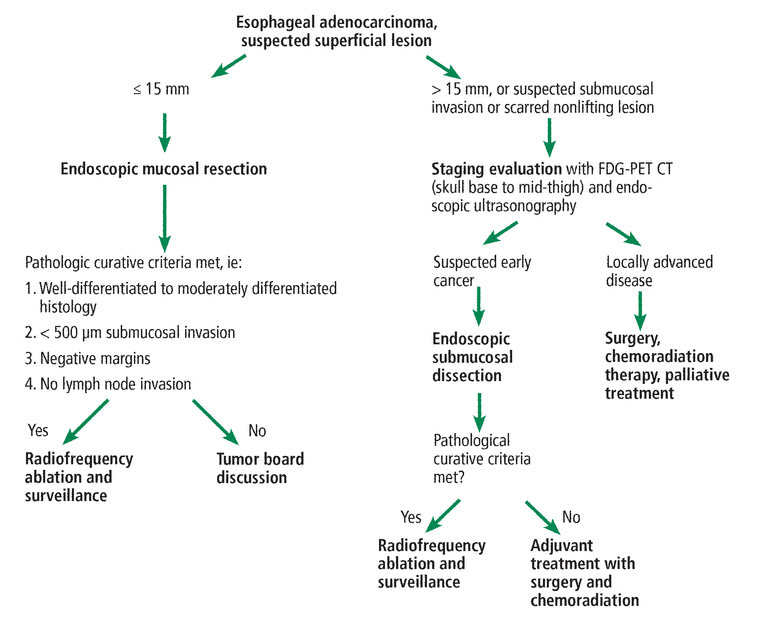
Our suggested care path for early esophageal adenocarcinoma is shown in Figure 4.
Our care path for early esophageal adenocarcinoma.
FDG-PET CT = 18-fluorodeoxyglucose positron-emission tomography with computed tomography
Surgery
For decades, the first-line treatment for early esophageal adenocarcinoma, including Barrett esophagus, has been open surgical resection. Technical advances in surgery such as robot-assisted minimally invasive esophagectomy, minimally invasive esophagectomy, and 3-dimensional imaging have improved recovery times and lymph node yield and have significantly decreased postoperative pain, intraoperative bleeding, and hospital length of stay.26
Minimally invasive approaches have become preferred, with long-term results that are not inferior to those of open esophagectomy. A study of more than 5,500 patients undergoing surgical resection showed a 90-day mortality rate of approximately 7%, which did not differ by surgical approach.27 However, mortality rates were lower for patients with T1a tumors (3.1%) and T1b tumors (6.0%).27
The role of surgical esophagectomy remains controversial in early T1a tumors with high-risk features such as poor differentiation and large size, due to high rates of perioperative mortality (3%–6%) and morbidity, with a similar risk of locoregional spread (4.2%).27 However, T1b tumors in otherwise healthy patients are considered for immediate esophagectomy due to the higher risk of lymph node metastasis (22%–28%).28 In a 2020 study, esophagectomy for T1b tumors was found to be associated with higher rates of overall survival and histologic remission compared with endoscopic resection.28 However, the patients treated endoscopically were older and had multiple comorbidities.
Postoperative surgical complications affect long-term mortality rates. Procedure-specific complications include conduit abnormalities, and recurrent laryngeal nerve injury; systemic complications include atrial fibrillation, myocardial infarction, and pneumonia. Long-term sequelae of esophagectomy include functional disorders such as dysphagia, delayed gastric emptying, reflux, and dumping syndrome. However, esophagectomy is usually well tolerated long-term with lifestyle changes such as eating frequent small-portion meals slowly and avoiding foods and beverages high in sugar.
Endoscopic surgery
Modern endoscopic techniques and devices have led to a shift to endoscopic treatment of early esophageal cancer rather than surgery, although not all early esophageal adenocarcinomas are amenable to curative endoscopic resection.
The esophageal architecture is unique in that the lymphatics penetrate through the muscularis mucosa and reach the lamina propria, leading to a theoretical risk of lymph node metastasis in early (T1a) tumors.29 Barrett esophagus-related cancer involving the mucosa is believed to have a small risk (1%– 2%) of lymph node metastasis, which increases with deeper invasion of the submucosa30:
7.5% with superficial submucosal invasion
10% with invasion in the middle third of the submucosa
45% with deep submucosal invasion.
Endoscopic resection can be considered in tumors at low risk for lymph node metastasis or in higher-risk tumors in patients who are medically unfit for surgery. The risks of perioperative death and of regional spread are between 3% and 4%.29,31 Therefore, it is important to weigh the risk of lymph node metastasis and the risk of morbidity and mortality of surgery in a patient before deciding the best therapeutic approach for early esophageal adenocarcinoma.
There are 2 main endoscopic resection techniques: endoscopic mucosal resection and endoscopic submucosal dissection.
Endoscopic mucosal resection can be performed by 2 main methods: cap-assisted endoscopic mucosal resection (Figure 5), in which a cap is attached to the tip of the endoscope to depress mucosal folds and allow better visualization, and banding.32 Esophageal endoscopic mucosal resection poses a 1.2% risk of bleeding, a 1% risk of stricture formation, and a low risk of perforation (0.2% to 1.3%).33 The safety, success rates, and procedural ease of endoscopic mucosal resection have established it as a mainstay in the treatment of early esophageal adenocarcinoma. However, for larger lesions, endoscopic mucosal resection requires removing the tumor in multiple pieces, which is associated with higher recurrence rates.
Band endoscopic mucosal resection of Barrett esophagus nodule. (A) Barrett esophagus nodule (arrow). (B) Resection bed after successful band endoscopic mucosal resection.
Endoscopic submucosal dissection can allow removal of even larger tumors in a single piece (en bloc) and is associated with higher rates of cure and a lower risk of recurrence, and it allows for precise histopathologic analysis.34–36
A prospective trial comparing endoscopic mucosal resection and endoscopic submucosal dissection for Barrett esophagus and esophageal adenocarcinoma found the en bloc resection rate to be 100% with endoscopic submucosal dissection, but only 15% with endoscopic mucosal resection.37 Likewise, a meta-analysis showed higher rates of R0 resection (margins free of neoplasia) (92.3% vs 52.7%) and lower rates of local recurrence (0.3% vs 11.5%) with endoscopic submucosal dissection than with endoscopic mucosal resection.38
Further information on these endoscopic techniques can be found in our earlier article in this Journal.39
Chemoradiation
Early esophageal adenocarcinoma (T1a, T1b) is primarily managed with endoscopic resection or surgery. However, recent evidence suggests that there may be a role for neoadjuvant (before resection) or adjuvant (after resection) chemoradiation therapy in early disease, particularly in patients with high-risk tumors (incomplete resection, positive deep margins, lymphovascular invasion, poorly differentiated tumors, tumors larger than 2 cm) who are medically unfit for surgery with lymph node dissection.28
The ChemoRadiotherapy for Oesophageal Cancer Followed by Surgery Study40 included patients with T1 to T3 and N0 to N1 resectable esophageal adenocarcinoma and showed higher survival rates when patients underwent neoadjuvant chemoradiation therapy before surgery. Of note, data on this topic are limited by studies that included only patients with esophageal squamous cell carcinoma.
Paclitaxel and carboplatin are commonly used with concurrent radiotherapy. Another combination that is increasingly being used is 5-fluorouracil and oxaliplatin concurrent with radiotherapy. An ongoing randomized trial is comparing these 2 adjuvant regimens for resectable esophageal adenocarcinoma.41
Radiotherapy alone (external-beam or brachytherapy) can be an option for patients over age 65 with esophageal adenocarcinoma who cannot undergo surgery or endoscopic therapy and concurrent chemotherapy. The data on radiation treatment alone are primarily from retrospective series in patients with esophageal squamous cell carcinoma. Poor surgical candidates who are definitively treated with chemoradiation therapy can have residual, recurrent, or metachronous disease. These patients can be managed with salvage endoscopic submucosal dissection or ablation therapy.
Further study is needed to explore the utility of neoadjuvant or adjuvant chemoradiation therapy in early esophageal adenocarcinoma.22
Adjuvant treatment after noncurative endoscopic resection
Patients with early esophageal adenocarcinoma are increasingly being treated with endoscopic resection. However, some resections are noncurative, with poor differentiation, lymphovascular invasion, deep submucosal invasion, or positive margins. These patients are at higher risk of lymph node metastasis and progressive disease.
Ideally, esophagectomy with or without adjuvant chemoradiation therapy is the treatment of choice for these patients. However, patients who have high-risk features after endoscopic resection and who are poor surgical candidates for definite esophagectomy with lymph node dissection can be referred for chemoradiation therapy.
A prospective trial in patients with T1a esophageal squamous cell carcinoma who underwent endoscopic submucosal dissection found a 3-year recurrence-free survival rate of 100% in those who received adjuvant radiotherapy and 85.3% in those who did not.42 Interestingly, no severe radiation adverse events were noted.
Surveillance following curative endoscopic resection
In esophageal adenocarcinoma, endoscopic resection is considered curative if the resection histology is well-differentiated to moderately differentiated with no lymph node invasion, with less than 500 μm submucosal invasion combined with negative lateral and deep margins.43 In comparison, squamous cell carcinoma endoscopic curative resection criteria include en bloc R0 resection of superficial lesions invading the lamina propria (T1a m2) with well-to-moderately differentiated histology with no lymphovascular invasion. En bloc R0 resection of a well-differentiated m3 or sm1 tumor (< 200 μm) without lymphovascular invasion has a low risk of lymph node metastasis, and these features are a relative indication for endoscopic submucosal dissection.43
Patients who undergo complete endoscopic resection of Barrett esophagus or esophageal adenocarcinoma are enrolled in a posttreatment surveillance program. Posttreatment surveillance is stratified based on postresection pathologic staging44:
For Barrett esophagus with high-grade dysplasia, upper endoscopy every 6 months for 2 years and then yearly is recommended.45
For T1a esophageal adenocarcinoma, endoscopic ultrasonography and CT can be considered, as these lesions have a 1% to 2% risk of lymph node metastasis. Surveillance consists of endoscopic ultrasonography every 6 months for 2 years, then endoscopic ultrasonography yearly and CT of the chest and abdomen yearly for 5 years.45
For higher-risk resections, surveillance includes endoscopic ultrasonography every 3 months for the first year followed by every 6 months for 1 year and then yearly. CT of the chest and abdomen is recommended at shorter intervals: every 6 months for the first year and yearly for the next 5 years.
THE BOTTOM LINE
Early esophageal adenocarcinoma is commonly diagnosed serendipitously in patients without symptoms undergoing upper endoscopy for other reasons. Due to the unique anatomy of the esophagus, even early esophageal adenocarcinoma has a risk of lymph node metastasis, and appropriate management is necessary.
For small esophageal adenocarcinoma lesions (ie, < 1.5 cm), multiple studies have shown endoscopic mucosal resection to be an effective strategy with good long-term results. For larger lesions or suspected deeper invasion or squamous cell carcinoma, a multidisciplinary approach is warranted. Endoscopic submucosal dissection can be effectively used to remove superficial tumors, despite their size or associated fibrosis. However, for lesions involving more than two-thirds of the circumference of the esophagus, there is a risk of esophageal stricture formation.
Patients with early esophageal adenocarcinoma and risk of lymph node metastasis are best treated with surgical resection, which allows for lymph node dissection, but many patients over age 65 or those with significant comorbidities may not be candidates for surgery. In these patients, endoscopic resection with adjuvant chemotherapy or radiotherapy can be considered. Some patients with early esophageal adenocarcinoma may not be candidates for either endoscopic or surgical resection owing to deep submucosal invasion, scarred disease, prior radiotherapy to the field, or severe comorbidities preventing anesthesia for procedure. In these patients, neoadjuvant radiotherapy, brachytherapy, chemotherapy, or a combination of these can be performed.
DISCLOSURES
Dr. Raja has disclosed intellectual property rights with Chromacode Inc and consulting for Smiths Medical. Dr. Kamath has disclosed consulting for Exelixis. Dr. Allende has disclosed acting as advisor or review panel participant for Incyte. Dr. Murthy has disclosed consulting and private ownership or partnership with Advanced Medical Solutions International. Dr. Bhatt has disclosed consulting for Aries Pharmaceuticals, Boston Scientific, Lumendi, and Medtronic, and intellectual property rights with Medtronic. The other authors report no relevant financial relationships which, in the context of their contributions, could be perceived as a potential conflict of interest.
- Copyright © 2022 The Cleveland Clinic Foundation. All Rights Reserved.