ABSTRACT
Vasomotor symptoms, also called hot flashes, hot flushes, and night sweats, are common during the menopause transition. Severe symptoms can substantially decrease quality of life. The authors first review current hormonal and nonhormonal therapies, then review evidence supporting the potential use of stellate ganglion block for managing vasomotor symptoms in perimenopausal and postmenopausal women.
Stellate ganglion block is a well-established treatment for pain.
Data indicate that stellate ganglion block reduces the frequency of vasomotor symptoms by 4% to 90%, with few adverse events.
Stellate ganglion block is currently recommended with caution owing to a lack of long-term clinical trial data on efficacy and safety.
Vasomotor symptoms (VMS), also called hot flashes, hot flushes, and night sweats, are common symptoms of menopause.1 They are described as moments of intense heat, usually accompanied by sweating and flushing in the upper body, including the head, neck, and upper torso,1 and they are associated with poor health outcomes and decreased quality of life. While hormonal therapies are the mainstay of treatment for VMS, there is a clear need for safe and effective nonhormonal treatment options for women who choose not to use hormone therapy and for those in whom hormone therapy is not effective.
Stellate ganglion block (SGB) is a promising alternative nonhormonal treatment. In this review, we describe the evidence supporting its use in the management of VMS in perimenopausal and postmenopausal women, particularly in those who have severe symptoms refractory to more conservative care.
THE EPIDEMIOLOGY OF VASOMOTOR SYMPTOMS
Approximately 60% to 80% of women experience VMS during the menopause transition,1,2 which averages 7 to 9 years, although some continue to have VMS in their 70s and 80s.2–5 These symptoms can be associated with a decrease in quality of life, often manifested as sleep disturbance, depression, and even mental exhaustion.1–6
Demographic and socioeconomic factors can affect VMS frequency and intensity. The Study of Women Across the Nation7 revealed that Black women have the highest prevalence and longest duration of VMS and are the most bothered by the symptoms. Women in lower socioeconomic positions were more likely to experience VMS. Also, those with a history of abuse or neglect, depression, anxiety, smoking, and early premenopausal onset of VMS have more severe and longer lasting VMS.7
Menopause-related symptoms also have a financial cost. Direct costs to the patient often lead to higher annual costs than other medical concerns in women in midlife.8 A 2015 article reported that healthcare costs for women with VMS were $1,346 higher than for their VMS-free counterparts, and women with VMS experienced lower productivity, with an indirect cost via work absenteeism, costing roughly $770 per year.9
TREATMENTS FOR VASOMOTOR SYMPTOMS
The most effective treatment for VMS is hormone therapy, either estrogen alone or combined with a progestogen.10 A Cochrane systematic review found that this therapy reduces the frequency and intensity of hot flashes associated with VMS by 75% to 79%.11 Hormone therapy has also been shown to be highly effective in early postmenopausal women.12 However, some women with VMS cannot use or choose not to use hormone therapy. Health conditions that are considered relative or absolute contraindications to hormone therapy include breast cancer, uninvestigated endometrial hyperplasia, hormone-responsive gynecologic cancers, unprovoked venous thromboembolism or thrombophilia, decompensated liver disease, myocardial infarction, stroke, and dementia.10
Nonhormonal options
A number of nonhormonal therapies for VMS are available.13
Selective serotonin reuptake inhibitors (SSRIs) and serotonin-norepinephrine reuptake inhibitors have been shown to reduce the frequency and severity of VMS in menopausal women.13,14 Specifically, paroxetine (the only nonhormonal medication for VMS approved by the US Food and Drug Administration), citalopram, and escitalopram have been shown to be effective and at lower doses than when used for anxiety and depression.14 Possible adverse effects include nausea and constipation, but these are less likely given the lower dose required to treat VMS.
Of note, women with a history of breast cancer who are also taking tamoxifen should not be prescribed SSRIs that inhibit CYP2D6 (eg, paroxetine, fluoxetine) because they can interfere with tamoxifen metabolism.14
Clonidine and gabapentin have also been shown to be effective in reducing VMS.15,16 Clonidine is limited in clinical use owing to a number of undesirable side effects such as weight gain, blurred vision, constipation, and orthostatic hypotension, and its modest rate of symptom improvement.17 Gabapentin may be most effective at treatment of nighttime symptoms since it can cause sleepiness. Oxybutynin, an antimuscarinic drug, has been found to be effective at reducing self-reported VMS frequency, with mild anticholinergic side effects such as dry mouth, constipation, and drowsiness.18 Neurokinin-3 receptor antagonists have also shown promise as non-hormonal therapy for VMS.19
Nonpharmaceutical options. The North American Menopause Society recommends the use of cognitive-behavioral therapy and hypnosis as evidence-based nonpharmaceutical options for VMS.13 Other options that have potential efficacy but lack definitive evidence include acupuncture20,21 and lifestyle changes such as wearing layered clothing, staying in cool atmospheres, and exercising.13,22. Herbal remedies and vitamin supplements (eg, black cohosh, vitamin E) have not been shown to be more effective than placebo.23 At present, there are more than 70 ongoing clinical trials of various treatments for VMS.24
STELLATE GANGLION BLOCK
SGB involves blockade of the sympathetic ganglia in the lower cervical and upper thoracic region using an anesthetic agent. It may also have a modulatory role in thermoregulatory areas of the brain.25
A range of indications
For more than 50 years, SGB has been a standard treatment for alleviating pain, including migraines, facial and upper-extremity pain, and complex regional pain syndrome. It has been used to treat immune and endocrine diseases affecting the head, neck, and upper extremities, as well as essential hypertension and hypotension, Behçet disease, Sjögren syndrome, myasthenia gravis, herpes zoster, gout, diabetes, and angina pectoris.26 SGB has also been used to treat pain and body temperature changes that do not traditionally respond to pain medication,27 hence the growing interest and research evaluating SGB in the treatment of VMS.
Current use for vasomotor symptoms in menopause
The North American Menopause Society currently recommends using SGB with caution as a nonhormonal treatment for moderate to severe VMS owing to its invasive nature and the lack of data from large long-term randomized trials.13 SGB is currently being used in women with severe VMS who cannot use hormone therapy or whose symptoms have not responded to other treatments. However, its use is limited by a lack of awareness, limited availability, and high cost (estimated to be $2,000 for a treatment course of 2 SGB injections).28,29
The procedure
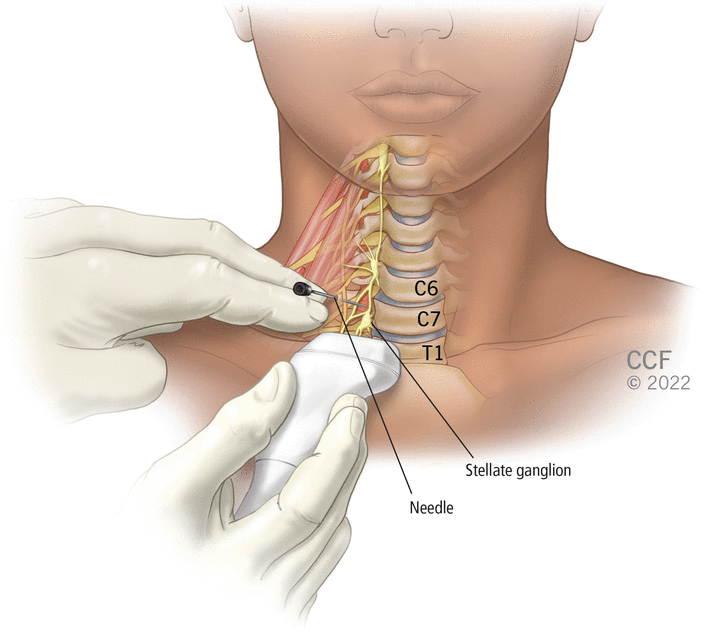
SGB involves injection of a local anesthetic such as lidocaine under fluoroscopic or ultrasonographic guidance. In clinical trials, both unilateral and bilateral approaches (including if refractory to unilateral treatment) have been used, although a right lateral approach seems to be preferred. Generally, the anesthetic is injected at the C6 or C7 vertebral level (Figure 1), targeting the sympathetic nerve chain that runs anterior to the neck of the first rib. The procedure takes about 30 minutes, with same-day patient discharge. Abatement of symptoms is highly variable in onset and impact.30
In stellate ganglion block, anesthetic is injected under ultrasonographic or fluoroscopic guidance into the stellate ganglion at the C6 or C7 vertebral level, targeting the sympathetic nerve chain that runs anterior to the transverse processes of the seventh cervical vertebra and the neck of the first rib.
WHAT HAVE STUDIES SHOWN?
Data on the efficacy of SGB for VMS have come from case reports, pilot studies, and randomized clinical trials. Table 1 presents detailed results from studies evaluating SGB for VMS in breast cancer survivors.17,31–36
Studies of stellate ganglion block for vasomotor symptoms in female breast cancer survivors
The first randomized, sham-controlled trial of fluoroscopy-guided SGB, published in 2014 by Walega et al,17 noted a 52% reduction in frequency of moderate to severe VMS symptoms from baseline to months 4 to 6 in the active-treatment group vs 4% in the control group (P < .001). The control group had an initial notable reduction in the frequency of VMS, but the SGB group had a significantly more sustained and effective impact. This reduction in frequency and intensity of VMS with SGB was similar to that described in previous nonrandomized intervention studies, with reductions varying from 34% to 90% over 4 weeks to several months after the procedure.31–34
In a 2018 clinical trial by Rahimzadeh et al,35 a group of 40 breast cancer survivors were randomly assigned to ultrasonography-guided SGB with 10 mL of 0.5% bupivacaine or to 6 weeks of oral therapy with 7.5 mg of paroxetine. A significant decrease in hot flash score (self-reported on the Sloan hot flash scoring scale)37 and sleep disturbance index (measured the Pittsburgh Sleep Quality Index)38 was identified in both groups, with no noticeable difference between the groups in efficacy, and with minimal (and fewer) side effects noted in the SGB group.
In a 2014 randomized controlled trial by Othman and Zaky,36 40 survivors of breast cancer were divided into 2 treatment groups, 1 group receiving SGB with 10 mL of 0.5% bupivacaine, and the other receiving 75 mg of pregabalin orally twice daily. Data were collected from baseline to 3 months, with VMS frequency reported via daily hot flash diary and monthly questionnaire. Hot flashes were self-reported on the Sloan hot flash scoring scale. This study showed a significant improvement in mild, moderate, and very severe hot flashes, and a decrease in frequency for both treatment groups. There were no significant differences shown between SGB and pregabalin, with no adverse events reported in either group.36
Case studies have also indicated tentative success with SGB for VMS. In a report of 6 patients by Lipov et al in 2005,39 SGB substantially decreased self-reported VMS. The initial SGB was shown to be successful based on 2 indicators: a positive test for Horner syndrome (ie, disrupted nerve pathway from brain to face and eye) and development of anhidrosis (ie, inability to sweat normally). However, results from this study describing 90% to 100% improvement in hot flashes have not been replicated in later studies.40
Other studies have reported a wide variation in hot flash improvements ranging from a 34% decrease in van Gastel et al32 to a 64% decrease in Haest et al,31 as well as in the methods used to measure improvement. The wide variability in hot flash reduction across studies may be explained by when the hot flashes were assessed (treatment effects can vary substantially over time), repetition and readministration of the treatment for increased efficacy, placebo effect, or the limitation-of-recall bias for self-reported hot flash diaries.
COMPLICATIONS ARE RARE BUT POTENTIALLY SIGNIFICANT
Complications of SGB are rare but can be significant and include central nervous system complications (eg, convulsions), vascular puncture, neural puncture, esophageal and tracheal puncture, spread of local anesthetic, pneumothorax, and allergic reactions.30 The published incidence of complications, predating the use of imaging guidance, is 1.7 per 1,000 procedures and correlates mostly with the intravascular injection of anesthesia that may lead to temporary seizures.17 With the increased use of imaging guidance, complications are less likely, although still relevant considering the critical structures in the injection area (eg, vertebral artery, internal carotid artery, inferior thyroid artery, other spinal nerves).17 Guidance with fluoroscopy or ultrasonography, monitoring cardiovascular function, and having resuscitative equipment available can minimize the risk of complications.30
HOW DOES STELLATE GANGLION BLOCK WORK?
The underlying mechanism for how SGB improves VMS is unclear. Lipov et al41 proposed that the mechanism likely involves peripheral vasodilation but noted that the wide range of indications for SGB (eg, pain treatments for migraines, atypical facial pain, upper extremity pain, complex regional pain syndrome, and, in Japan, diseases of the immune and endocrine systems) may indicate a more complicated mechanism of action. In a rat study, Westerhaus and Loewy42 used pseudorabies virus injections to find the neural pathway of stellate ganglion block and uncovered connections to the hypothalamus and amygdala, supporting hypotheses that the stellate ganglia are intricately involved with modulating temperature and factors that influence pain.42 The unifying mechanism may be through nerve growth factor, which is involved in cell differentiation, survival, and apoptosis, increasing brain norepinephrine in various illnesses and conditions, as well as through a possible reduction in the concentration of nerve growth factor and norepinephrine to deactivate these states.41 Others have hypothesized that SGB results in changes in voltage-gated sodium channels of peripheral nerves and central response by spinal feedback loops, thus decreasing VMS.43 More research is needed to clarify the mechanisms by which SGB treats VMS.
THE BOTTOM LINE
VMS is common and is associated with decreased quality of life in perimenopausal and postmenopausal women. Nonhormonal treatment options for VMS that are safe and effective are important for women who cannot use or choose not to use hormone therapy. SGB is a promising treatment. Based on existing data, it can be considered with caution in patients with severe VMS whose symptoms are refractory to conservative care, who can afford the treatment, and who have access to this service. Although cost data are limited, preliminary analyses indicate that SGB could balance out the cost of hormone therapy, and some insurance companies cover the cost of SGB in VMS.44
Making more practitioners aware of SGB as a treatment option will be important for its adoption in clinical practice. However, the wide variability in study results highlights the need for robust long-term randomized clinical trials to evaluate the neuromodulatory mechanisms of SGB before the procedure can be widely endorsed for VMS.
DISCLOSURES
Dr. Kling reports consulting for Procter & Gamble and for Triangle Insights Group. The other authors report no relevant financial relationships which, in the context of their contributions, could be perceived as a potential conflict of interest.
- Copyright © 2022 The Cleveland Clinic Foundation. All Rights Reserved.